CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis
- PMID: 18042724
- PMCID: PMC2148337
- DOI: 10.1073/pnas.0705147104
CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis
Abstract
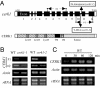
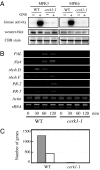
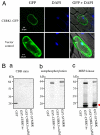
Chitin is a major component of fungal cell walls and serves as a microbe-associated molecular pattern (MAMP) for the detection of various potential pathogens in innate immune systems of both plants and animals. We recently showed that chitin elicitor-binding protein (CEBiP), plasma membrane glycoprotein with LysM motifs, functions as a cell surface receptor for chitin elicitor in rice. The predicted structure of CEBiP does not contain any intracellular domains, suggesting that an additional component(s) is required for signaling through the plasma membrane into the cytoplasm. Here, we identified a receptor-like kinase, designated CERK1, which is essential for chitin elicitor signaling in Arabidopsis. The KO mutants for CERK1 completely lost the ability to respond to the chitin elicitor, including MAPK activation, reactive oxygen species generation, and gene expression. Disease resistance of the KO mutant against an incompatible fungus, Alternaria brassicicola, was partly impaired. Complementation with the WT CERK1 gene showed cerk1 mutations were responsible for the mutant phenotypes. CERK1 is a plasma membrane protein containing three LysM motifs in the extracellular domain and an intracellular Ser/Thr kinase domain with autophosphorylation/myelin basic protein kinase activity, suggesting that CERK1 plays a critical role in fungal MAMP perception in plants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Heath MC. Curr Opin Plant Biol. 2000;3:315–319. - PubMed
-
- Nürnberger T, Brunner F, Kemmerling B, Piater L. Immunol Rev. 2004;198:249–266. - PubMed
-
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Cell. 2006;124:803–814. - PubMed
-
- Nürnberger T, Lipka V. Mol Plant Pathol. 2005;6:335–345. - PubMed
-
- Jones JD, Dangl JL. Nature. 2006;444:323–329. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

