Epidermal growth factor receptor juxtamembrane region regulates allosteric tyrosine kinase activation
- PMID: 18042729
- PMCID: PMC2148274
- DOI: 10.1073/pnas.0703854104
Epidermal growth factor receptor juxtamembrane region regulates allosteric tyrosine kinase activation
Abstract
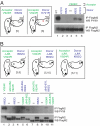
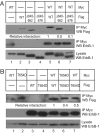
Structural studies of the extracellular and tyrosine kinase domains of the epidermal growth factor receptor (ErbB-1) provide considerable insight into facets of the receptor activation mechanism, but the contributions of other regions of ErbB-1 have not been ascertained. This study demonstrates that the intracellular juxtamembrane (JM) region plays a vital role in the kinase activation mechanism. In the experiments described herein, the entire ErbB-1 intracellular domain (ICD) has been expressed in mammalian cells to explore the significance of the JM region in kinase activity. Deletion of the JM region (DeltaJM) results in a severe loss of ICD tyrosine phosphorylation, indicating that this region is required for maximal activity of the tyrosine kinase domain. Coexpression of DeltaJM and dimerization-deficient kinase domain ICD mutants revealed that the JM region is indispensable for allosteric kinase activation and productive monomer interactions within a dimer. Studies with the intact receptor confirmed the role of the JM region in kinase activation. Within the JM region, Thr-654 is a known protein kinase C (PKC) phosphorylation site that modulates kinase activity in the context of the intact ErbB-1 receptor; yet, the mechanism is not known. Whereas a T654A mutation promotes increased ICD tyrosine phosphorylation, the phosphomimetic T654D mutant generates a 50% reduction in ICD tyrosine phosphorylation. Similar to the DeltaJM mutants, the T654D mutant ICD failed to interact with a wild-type monomer. This study reveals an integral role for the intracellular JM region of ErbB-1 in allosteric kinase activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jae Song, Kil CC. J Cell Physiol. 2000;185:47–60. - PubMed
-
- Kil SJ, Hobert M, Carlin C. J Biol Chem. 1999;274:3141–3150. - PubMed
-
- He C, Hobert M, Friend L, Carlin C. J Biol Chem. 2002;277:38284–38293. - PubMed
-
- Lin S-Y, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung M-C. Nat Cell Biol. 2001;3:802–808. - PubMed
-
- Martin-Nieto J, Villalobo A. Biochemistry. 1998;37:227–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

