Potent activity of the HIV-1 maturation inhibitor bevirimat in SCID-hu Thy/Liv mice
- PMID: 18043758
- PMCID: PMC2080775
- DOI: 10.1371/journal.pone.0001251
Potent activity of the HIV-1 maturation inhibitor bevirimat in SCID-hu Thy/Liv mice
Abstract
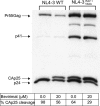
Background: The HIV-1 maturation inhibitor, 3-O-(3',3'-dimethylsuccinyl) betulinic acid (bevirimat, PA-457) is a promising drug candidate with 10 nM in vitro antiviral activity against multiple wild-type (WT) and drug-resistant HIV-1 isolates. Bevirimat has a novel mechanism of action, specifically inhibiting cleavage of spacer peptide 1 (SP1) from the C-terminus of capsid which results in defective core condensation.
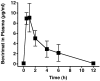
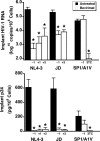
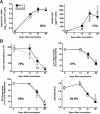
Methods and findings: Oral administration of bevirimat to HIV-1-infected SCID-hu Thy/Liv mice reduced viral RNA by >2 log(10) and protected immature and mature T cells from virus-mediated depletion. This activity was observed at plasma concentrations that are achievable in humans after oral dosing, and bevirimat was active up to 3 days after inoculation with both WT HIV-1 and an AZT-resistant HIV-1 clinical isolate. Consistent with its mechanism of action, bevirimat caused a dose-dependent inhibition of capsid-SP1 cleavage in HIV-1-infected human thymocytes obtained from these mice. HIV-1 NL4-3 with an alanine-to-valine substitution at the N-terminus of SP1 (SP1/A1V), which is resistant to bevirimat in vitro, was also resistant to bevirimat treatment in the mice, and SP1/AIV had replication and thymocyte kinetics similar to that of WT NL4-3 with no evidence of fitness impairment in in vivo competition assays. Interestingly, protease inhibitor-resistant HIV-1 with impaired capsid-SP1 cleavage was hypersensitive to bevirimat in vitro with a 50% inhibitory concentration 140 times lower than for WT HIV-1.
Conclusions: These results support further clinical development of this first-in-class maturation inhibitor and confirm the usefulness of the SCID-hu Thy/Liv model for evaluation of in vivo antiretroviral efficacy, drug resistance, and viral fitness.
Conflict of interest statement
Figures




Similar articles
-
Resistance to Second-Generation HIV-1 Maturation Inhibitors.J Virol. 2019 Mar 5;93(6):e02017-18. doi: 10.1128/JVI.02017-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30567982 Free PMC article.
-
In vitro resistance to the human immunodeficiency virus type 1 maturation inhibitor PA-457 (Bevirimat).J Virol. 2006 Nov;80(22):10957-71. doi: 10.1128/JVI.01369-06. Epub 2006 Sep 6. J Virol. 2006. PMID: 16956950 Free PMC article.
-
Alkyl Amine Bevirimat Derivatives Are Potent and Broadly Active HIV-1 Maturation Inhibitors.Antimicrob Agents Chemother. 2015 Oct 19;60(1):190-7. doi: 10.1128/AAC.02121-15. Print 2016 Jan. Antimicrob Agents Chemother. 2015. PMID: 26482309 Free PMC article.
-
Bevirimat: a novel maturation inhibitor for the treatment of HIV-1 infection.Antivir Chem Chemother. 2008;19(3):107-13. doi: 10.1177/095632020801900301. Antivir Chem Chemother. 2008. PMID: 19024627 Review.
-
HIV-1 Maturation: Lessons Learned from Inhibitors.Viruses. 2020 Aug 26;12(9):940. doi: 10.3390/v12090940. Viruses. 2020. PMID: 32858867 Free PMC article. Review.
Cited by
-
Developmental regulation of P-glycoprotein activity within thymocytes results in increased anti-HIV protease inhibitor activity.J Leukoc Biol. 2011 Oct;90(4):653-60. doi: 10.1189/jlb.0111-009. Epub 2011 Apr 19. J Leukoc Biol. 2011. PMID: 21504949 Free PMC article.
-
Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach.J Nat Prod. 2010 Mar 26;73(3):500-16. doi: 10.1021/np900821e. J Nat Prod. 2010. PMID: 20187635 Free PMC article. Review.
-
Oral administration of the nucleoside EFdA (4'-ethynyl-2-fluoro-2'-deoxyadenosine) provides rapid suppression of HIV viremia in humanized mice and favorable pharmacokinetic properties in mice and the rhesus macaque.Antimicrob Agents Chemother. 2015 Jul;59(7):4190-8. doi: 10.1128/AAC.05036-14. Epub 2015 May 4. Antimicrob Agents Chemother. 2015. PMID: 25941222 Free PMC article.
-
Enhancing protein backbone binding--a fruitful concept for combating drug-resistant HIV.Angew Chem Int Ed Engl. 2012 Feb 20;51(8):1778-802. doi: 10.1002/anie.201102762. Epub 2012 Jan 31. Angew Chem Int Ed Engl. 2012. PMID: 22290878 Free PMC article. Review.
-
Effect of white mange mixture in a murine model of psoriasis.Exp Ther Med. 2019 Aug;18(2):881-887. doi: 10.3892/etm.2019.7641. Epub 2019 Jun 4. Exp Ther Med. 2019. PMID: 31384318 Free PMC article.
References
-
- Pillay D, Green H, Matthias R, Dunn D, Phillips A, et al. Estimating HIV-1 drug resistance in antiretroviral-treated individuals in the United Kingdom. J Infect Dis. 2005;192:967–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

