Outcomes in COPD patients receiving tiotropium or salmeterol plus treatment with inhaled corticosteroids
- PMID: 18044688
- PMCID: PMC3321723
Outcomes in COPD patients receiving tiotropium or salmeterol plus treatment with inhaled corticosteroids
Abstract
Patients with COPD are frequently prescribed inhaled corticosteroids (ICS); however, it is unclear whether the treatment with ICS might modify responses to inhaled bronchodilators. Two 6-month, randomized, placebo-controlled, double-blind, double-dummy, parallel-group studies of tiotropium 18 microg once daily, compared with salmeterol, 50 microg bid, had been conducted in patients with moderate-to-severe COPD. Efficacy was assessed by spirometry, transition dyspnea index (TDI), St. George's Respiratory Questionnaire (SGRQ), and exacerbations. Data from both studies were combined to form subgroups with regard to concurrent use of ICS. 796 patients receiving ICS were separately analyzed from 390 patients not receiving ICS. Mean age was 64 years, and pre-bronchodilator FEV1 was 1.06 L (ICS group) and 1.13 L (non-ICS group). Both bronchodilators increased morning mean +/- SE pre-dose FEV1 compared with placebo (ICS groups: tiotropium 110 +/- 20 mL, salmeterol 80 +/- 20 mL; non-ICS groups: tiotropium 150 +/- 30 mL, salmeterol 110 +/- 30 mL; p > 0.05 for tiotropium vs salmeterol). Improvements in TDI and SGRQ and frequency of exacerbations also tended to be more profound for tiotropium. Treatment with tiotropium in patients with moderate-to-severe COPD was superior to salmeterol in lung function, irrespective of concurrent use of ICS.
Figures
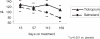


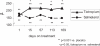

Comment in
-
Which bronchodilator in COPD?Int J Chron Obstruct Pulmon Dis. 2007;2(2):93-4. Int J Chron Obstruct Pulmon Dis. 2007. PMID: 18046837 Free PMC article. No abstract available.
References
-
- Aaron SD, Vandemheen KL, Fergusson D, et al. for the Canadian Thoracic Society/Canadian Respiratory Clinical Research Consortium Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545–55. - PubMed
-
- Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med. 2002;113(1):59–65. - PubMed
-
- Barnes PJ. Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:342–344. - PubMed
-
- Barnes P. The role of anticholinergics in chronic obstructive pulmonary disease. Am J Med. 2004;117:24S–32S. - PubMed
-
- Barnes P, Adcock I, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005;25:552–63. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

