An LRP5 receptor with internal deletion in hyperparathyroid tumors with implications for deregulated WNT/beta-catenin signaling
- PMID: 18044981
- PMCID: PMC2082644
- DOI: 10.1371/journal.pmed.0040328
An LRP5 receptor with internal deletion in hyperparathyroid tumors with implications for deregulated WNT/beta-catenin signaling
Abstract
Background: Hyperparathyroidism (HPT) is a common endocrine disorder with incompletely understood etiology, characterized by enlarged hyperactive parathyroid glands and increased serum concentrations of parathyroid hormone and ionized calcium. We have recently reported activation of the Wnt signaling pathway by accumulation of beta-catenin in all analyzed parathyroid tumors from patients with primary HPT (pHPT) and in hyperplastic parathyroid glands from patients with uremia secondary to HPT (sHPT). Mechanisms that may account for this activation have not been identified, except for a few cases of beta-catenin (CTNNB1) stabilizing mutation in pHPT tumors.
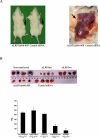
Methods and findings: Reverse transcription PCR and Western blot analysis showed expression of an aberrantly spliced internally truncated WNT coreceptor low-density lipoprotein receptor-related protein 5 (LRP5) in 32 out of 37 pHPT tumors (86%) and 20 out of 20 sHPT tumors (100%). Stabilizing mutation of CTNNB1 and expression of the internally truncated LRP5 receptor was mutually exclusive. Expression of the truncated LRP5 receptor was required to maintain the nonphosphorylated active beta-catenin level, transcription activity of beta-catenin, MYC expression, parathyroid cell growth in vitro, and parathyroid tumor growth in a xenograft severe combined immunodeficiency (SCID) mouse model. WNT3 ligand and the internally truncated LRP5 receptor strongly activated transcription, and the internally truncated LRP5 receptor was insensitive to inhibition by DKK1.
Conclusions: The internally truncated LRP5 receptor is strongly implicated in deregulated activation of the WNT/beta-catenin signaling pathway in hyperparathyroid tumors, and presents a potential target for therapeutic intervention.
Conflict of interest statement
Figures





References
-
- Marx SJ. Hyperparathyroid and hypoparathyroid disorders. N Engl J Med. 2000;343:1863–1875. - PubMed
-
- Arnold A, Shattuck TM, Mallya SM, Krebs LJ, Costa J, et al. Molecular pathogenesis of primary hyperparathyroidism. J Bone Miner Res. 2002;17(Suppl 2):N30–N36. - PubMed
-
- Åkerström G, Hellman P. Primary hyperparathyroidism. Curr Opin Oncol. 2004;16:1–7. - PubMed
-
- Åkerström G, Hellman P, Hessman O, Segersten U, Westin G. Parathyroid glands in calcium regulation and human disease. Ann N Y Acad Sci. 2005;1040:53–58. - PubMed
-
- Björklund P, Åkerström G, Westin G. Accumulation of nonphosphorylated beta-catenin and c-myc in primary and uremic secondary hyperparathyroid tumors. J Clin Endocrinol Metab. 2007;92:338–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

