Epithelial: lamina propria lymphocyte interactions promote epithelial cell differentiation
- PMID: 18045591
- PMCID: PMC2975902
- DOI: 10.1053/j.gastro.2007.10.022
Epithelial: lamina propria lymphocyte interactions promote epithelial cell differentiation
Abstract
Background & aims: Intestinal lymphoepithelial interactions occur in the epithelium and the subepithelial space. We asked whether normal, Crohn's disease (CD), or ulcerative colitis (UC) lamina propria lymphocytes (LPL) could promote intestinal epithelial cell (IEC) growth and differentiation.
Methods: T84 cells were cocultured with isolated LPL. IECs were then lysed and subjected to measurement of intestinal alkaline phosphatase (IAP) activity; Western blot analysis for MAPK and Akt activation; and real-time polymerase chain reaction to assess caudal-related homeoprotein 2 (CDX2) messenger RNA (mRNA) levels. Tissue sections were immunostained for evidence of mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) activation, CDX2, and IAP; and CDX2 mRNA expression was assessed in human colonic biopsy specimens.
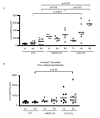
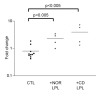
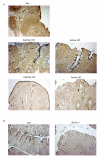
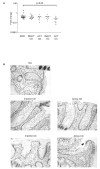
Results: IAP activity was increased in T84 cells cocultured for 8 days with normal LPL (P < .05) and even greater with CD LPL (P < .001). Crypt IECs in active CD mucosa expressed IAP ex vivo. Phospho-MAPK (extracellular signal-regulated kinase 1/2, p38, and c-Jun-N-terminal kinase) and phospho-Akt were seen as early as 30 minutes after coculture. MAPK activation was greatest in T84 cells cocultured with CD LPL. There was a specific increase in Phospho-p38 MAPK and Phospho-Akt staining in the nuclei of crypt IECs in active vs inactive CD, normal mucosa, and UC mucosa. CDX2 mRNA expression was increased in CD LPL cocultured T84 cells, which did not correlate with CDX2 protein localization ex vivo.
Conclusions: There is cross talk between LPL and IECs, which leads to IEC differentiation. The differentiation is accelerated in CD mucosa.
Figures


References
-
- Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells - polarity and complexity. Immunol Today. 2000;21:123–8. - PubMed
-
- Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123:1516–26. - PubMed
-
- Allez M, Brimnes J, Shao L, Dotan I, Nakazawa A, Mayer L. Activation of a unique population of CD8(+) T cells by intestinal epithelial cells. Ann N Y Acad Sci. 2004;1029:22–35. - PubMed
-
- Allez M, Mayer L. Regulatory T cells: peace keepers in the gut. Inflamm Bowel Dis. 2004;10:666–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

