A T-cell receptor associated with naturally occurring human tumor immunity
- PMID: 18045792
- PMCID: PMC2141910
- DOI: 10.1073/pnas.0704336104
A T-cell receptor associated with naturally occurring human tumor immunity
Abstract
The onconeural antigens appear to serve as tumor rejection antigens in the paraneoplastic neurologic disorders. Here, we used an unbiased peptide binding screen, followed by studies in HLA-A2.1 transgenic mice to identify naturally processed HLA-A2.1 restricted epitopes of the paraneoplastic cerebellar degeneration breast/ovarian cancer antigen cdr2. These mice were used to clone high-avidity cdr2-specific CD8(+) T cells that recognize human tumor cells presenting endogenously loaded MHC class I-cdr2 peptide. T cells with this specificity were detected in the peripheral blood of two HLA-A2.1(+) paraneoplastic cerebellar degeneration patients. We cloned T cell receptor (TCR) alpha and beta genes from cdr2-specific T cells; electroporation of RNA encoding this TCR turned nonreactive donor T cells into efficient killers of human cdr2-expressing tumor cells. Cloned cdr2-specific TCR genes provide a clinically relevant means for immunologic targeting of human gynecologic cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
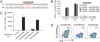
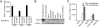

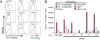

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

