Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger
- PMID: 18045900
- PMCID: PMC6673405
- DOI: 10.1523/JNEUROSCI.3467-07.2007
Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger
Abstract
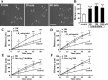
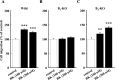
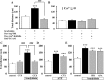
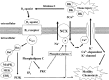
Bradykinin (BK) is produced and acts at the site of injury and inflammation. In the CNS, migration of microglia toward the lesion site plays an important role pathologically. In the present study, we investigated the effect of BK on microglial migration. Increased motility of cultured microglia was mimicked by B1 receptor agonists and markedly inhibited by a B1 antagonist but not by a B2 receptor antagonist. BK induced chemotaxis in microglia isolated from wild-type and B2-knock-out mice but not from B1-knock-out mice. BK-induced motility was not blocked by pertussis toxin but was blocked by chelating intracellular Ca2+ or by low extracellular Ca2+, implying that Ca2+ influx is prerequisite. Blocking the reverse mode of Na+/Ca2+ exchanger (NCX) completely inhibited BK-induced migration. The involvement of NCX was further confirmed by using NCX+/- mice; B1-agonist-induced motility and chemotaxis was decreased compared with that in NCX+/+ mice. Activation of NCX seemed to be dependent on protein kinase C and phosphoinositide 3-kinase, and resultant activation of intermediate-conductance (IK-type) Ca2+-dependent K+ currents (I(K(Ca))) was activated. Despite these effects, BK did not activate microglia, as judged from OX6 staining. Using in vivo lesion models and pharmacological injection to the brain, it was shown that microglial accumulation around the lesion was also dependent on B1 receptors and I(K(Ca)). These observations support the view that BK functions as a chemoattractant by using the distinct signal pathways in the brain and, thus, attracts microglia to the lesion site in vivo.
Figures





Comment in
-
Bradykinin: a microglia attractant in vivo?J Neurosci. 2008 Apr 2;28(14):3531-2. doi: 10.1523/JNEUROSCI.0374-08.2008. J Neurosci. 2008. PMID: 18385310 Free PMC article. No abstract available.
References
-
- Busser BW, Hammond TG, Bjorling DE, Saban R. Lipopolysaccharide upregulates bradykinin 1 receptors in the isolated mouse bladder. J Urol. 1998;160:2267–2273. - PubMed
-
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. - PubMed
-
- Delmas P, Wananerbercq N, Abogadie FC, Mistry M, Brown DA. Signaling microdomains define the specificity of receptor-mediated InsP3 pathways in neurons. Neuron. 2002;14:209–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
