Cdc42 regulates cofilin during the establishment of neuronal polarity
- PMID: 18045906
- PMCID: PMC6673401
- DOI: 10.1523/JNEUROSCI.3322-07.2007
Cdc42 regulates cofilin during the establishment of neuronal polarity
Abstract
The establishment of polarity is an essential process in early neuronal development. Although a number of molecules controlling neuronal polarity have been identified, genetic evidence about their physiological roles in this process is mostly lacking. We analyzed the consequences of loss of Cdc42, a central regulator of polarity in multiple systems, on the polarization of mammalian neurons. Genetic ablation of Cdc42 in the brain led to multiple abnormalities, including striking defects in the formation of axonal tracts. Neurons from the Cdc42 null animals sprouted neurites but had a strongly suppressed ability to form axons both in vivo and in culture. This was accompanied by disrupted cytoskeletal organization, enlargement of the growth cones, and inhibition of filopodial dynamics. Axon formation in the knock-out neurons was rescued by manipulation of the actin cytoskeleton, indicating that the effects of Cdc42 ablation are exerted through modulation of actin dynamics. In addition, the knock-outs showed a specific increase in the phosphorylation (inactivation) of the Cdc42 effector cofilin. Furthermore, the active, nonphosphorylated form of cofilin was enriched in the axonal growth cones of wild-type, but not of mutant, neurons. Importantly, cofilin knockdown resulted in polarity defects quantitatively analogous to the ones seen after Cdc42 ablation. We conclude that Cdc42 is a key regulator of axon specification, and that cofilin is a physiological downstream effector of Cdc42 in this process.
Figures

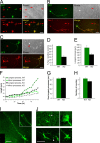



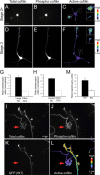
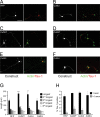
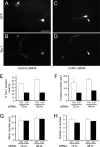
References
-
- Abe H, Ohshima S, Obinata T. A cofilin-like protein is involved in the regulation of actin assembly in developing skeletal muscle. J Biochem (Tokyo) 1989;106:696–702. - PubMed
-
- Abe T, Kato M, Miki H, Takenawa T, Endo T. Small GTPase Tc10 and its homologue RhoT induce N-WASP-mediated long process formation and neurite outgrowth. J Cell Sci. 2003;116:155–168. - PubMed
-
- Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem. 1995;270:17582–17587. - PubMed
-
- Andersen SS, Bi GQ. Axon formation: a molecular model for the generation of neuronal polarity. BioEssays. 2000;22:172–179. - PubMed
-
- Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
