Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice
- PMID: 18045908
- PMCID: PMC2747091
- DOI: 10.1523/JNEUROSCI.2284-07.2007
Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice
Abstract
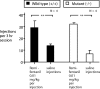
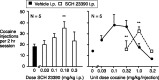
Evidence suggests a critical role for dopamine in the reinforcing effects of cocaine in rats and primates. However, self-administration has been less often studied in the mouse species, and, to date, "knock-out" of individual dopamine-related genes in mice has not been reported to reduce the reinforcing effects of cocaine. We studied the dopamine D1 receptor and cocaine self-administration in mice using a combination of gene-targeted mutation and pharmacological tools. Two cohorts with varied breeding and experimental histories were tested, and, in both cohorts, there was a significant decrease in the number of D1 receptor knock-out mice that met criteria for acquisition of cocaine self-administration (2 of 23) relative to wild-type mice (27 of 32). After extinction of responding with saline self-administration, dose-response studies showed that cocaine reliably and dose dependently maintained responding greater than saline in all wild-type mice but in none of the D1 receptor knock-out mice. The D1-like agonist SKF 82958 (2,3,4,5,-tetrahydro-6-chloro-7,8-dihydroxy-1-phenyl-1H-3-benzazepine hydrobromide) and the D2-like agonist quinelorane both functioned as positive reinforcers in wild-type mice but not in D1 receptor mutant mice, whereas food and intravenous injections of the opioid agonist remifentanil functioned as positive reinforcers in both genotypes. Finally, pretreatment with the D1-like antagonist SCH 23390 [R-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepine-7-01] produced surmountable antagonism of the reinforcing effects of cocaine in the commonly used strain C57BL/6J. We conclude that D1 receptor knock-out mice do not reliably self-administer cocaine and that the D1 receptor is critical for the reinforcing effects of cocaine and other dopamine agonists, but not food or opioids, in mice.
Figures
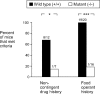

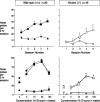
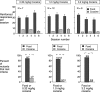

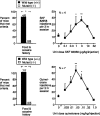
Similar articles
-
Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat.Neuroscience. 2006 Oct 13;142(2):451-61. doi: 10.1016/j.neuroscience.2006.06.004. Epub 2006 Jul 14. Neuroscience. 2006. PMID: 16844308
-
Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats.Neuropharmacology. 2004;47 Suppl 1:256-73. doi: 10.1016/j.neuropharm.2004.07.007. Neuropharmacology. 2004. PMID: 15464142
-
Modulation of heroin and cocaine self-administration by dopamine D1- and D2-like receptor agonists in rhesus monkeys.J Pharmacol Exp Ther. 2007 Jun;321(3):1135-43. doi: 10.1124/jpet.107.120766. Epub 2007 Mar 9. J Pharmacol Exp Ther. 2007. PMID: 17351103
-
Locomotor activity and cocaine-seeking behavior during acquisition and reinstatement of operant self-administration behavior in rats.Behav Brain Res. 2005 May 28;160(2):250-9. doi: 10.1016/j.bbr.2004.12.005. Epub 2005 Jan 12. Behav Brain Res. 2005. PMID: 15863221
-
Contributions to drug abuse research of Steven R. Goldberg's behavioral analysis of stimulus-stimulus contingencies.Psychopharmacology (Berl). 2016 May;233(10):1921-32. doi: 10.1007/s00213-015-4149-x. Epub 2015 Nov 13. Psychopharmacology (Berl). 2016. PMID: 26564234 Free PMC article. Review.
Cited by
-
Delineating the Psychic Structure of Substance Use and Addictions, from Neurobiology to Clinical Implications: Ten Years Later.J Clin Med. 2020 Jun 18;9(6):1913. doi: 10.3390/jcm9061913. J Clin Med. 2020. PMID: 32570932 Free PMC article.
-
An optimized procedure for robust volitional cocaine intake in mice.Exp Clin Psychopharmacol. 2021 Aug;29(4):319-333. doi: 10.1037/pha0000399. Epub 2020 Jul 13. Exp Clin Psychopharmacol. 2021. PMID: 32658535 Free PMC article.
-
Molecular Imaging of Opioid and Dopamine Systems: Insights Into the Pharmacogenetics of Opioid Use Disorders.Front Psychiatry. 2019 Sep 18;10:626. doi: 10.3389/fpsyt.2019.00626. eCollection 2019. Front Psychiatry. 2019. PMID: 31620026 Free PMC article. Review.
-
Individual differences in the neuropsychopathology of addiction.Dialogues Clin Neurosci. 2017 Sep;19(3):217-229. doi: 10.31887/DCNS.2017.19.3/gkoob. Dialogues Clin Neurosci. 2017. PMID: 29302219 Free PMC article. Review.
-
Effects of β-Phenylethylamine on Psychomotor, Rewarding, and Reinforcing Behaviors and Affective State: The Role of Dopamine D1 Receptors.Int J Mol Sci. 2021 Aug 31;22(17):9485. doi: 10.3390/ijms22179485. Int J Mol Sci. 2021. PMID: 34502393 Free PMC article.
References
-
- Barr JE, Holmes DB, Ryan LJ, Sharpless SK. Techniques for the chronic cannulation of the jugular vein in mice. Pharmacol Biochem Behav. 1979;11:115–118. - PubMed
-
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food-maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–273. - PubMed
-
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D1 and D2 antagonists. Behav Pharmacol. 1990;1:355–363. - PubMed
-
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. - PubMed
-
- Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA013786/DA/NIDA NIH HHS/United States
- R01 DA017323/DA/NIDA NIH HHS/United States
- R01-NS32595/NS/NINDS NIH HHS/United States
- R01-DA17323/DA/NIDA NIH HHS/United States
- R01-DA04398/DA/NIDA NIH HHS/United States
- P01 DA014528/DA/NIDA NIH HHS/United States
- R01 DA014644/DA/NIDA NIH HHS/United States
- R01 DA004398/DA/NIDA NIH HHS/United States
- R29-DA12142/DA/NIDA NIH HHS/United States
- R29 DA012142/DA/NIDA NIH HHS/United States
- T32 DA007252/DA/NIDA NIH HHS/United States
- P01-DA14528/DA/NIDA NIH HHS/United States
- R01-DA13786/DA/NIDA NIH HHS/United States
- R01-DA14644/DA/NIDA NIH HHS/United States
- T32-DA07252/DA/NIDA NIH HHS/United States
- R29-DA11284/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
