Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus
- PMID: 18045923
- PMCID: PMC6673415
- DOI: 10.1523/JNEUROSCI.3502-07.2007
Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus
Abstract
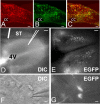
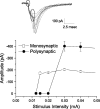
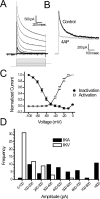
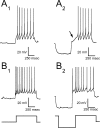
Brainstem A2/C2 neurons are catecholamine (CA) neurons within the solitary tract nucleus (NTS) that influence many homeostatic functions, including cardiovascular reflexes, food intake, and stress. Because NTS is a major interface between sensory visceral afferents and the CNS, NTS CA neurons are ideally suited to coordinate complex responses by their projections to multiple brain regions. To test how NTS CA neurons process visceral afferent information carried by solitary tract (ST) afferents, we identified CA neurons using transgenic mice expressing TH-EGFP (enhanced green fluorescent protein under the control of the tyrosine hydroxylase promoter) and recorded synaptic responses to ST activation in horizontal slices. ST shocks evoked large-amplitude, short-latency, glutamatergic EPSCs (ST-EPSCs) in 90% of NTS CA neurons. Within neurons, ST-EPSCs had constant latency, rarely failed, and depressed substantially at high ST frequencies, indicating that NTS CA neurons receive direct monosynaptic connections from afferent terminals. NTS CA neurons received direct ST inputs from only one or two afferent fibers, with one-half also receiving smaller amplitude indirect inputs. Up to 90% of ST shocks evoked action potentials in NTS CA neurons. However, transmission of sensory afferent information through NTS CA neurons critically depended on the expression of an A-type potassium current (I(KA)), which when active attenuated ST-activated action potentials to a 37% success rate. The satiety peptide, cholecystokinin, presynaptically facilitated glutamate transmission in one-half of NTS CA neurons. Thus, NTS CA neurons are directly driven by visceral afferents with output being modulated by presynaptic peptide receptors and postsynaptic potassium channels.
Figures




References
-
- Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. - PubMed
-
- Andresen MC, Yang M. Dynamics of sensory afferent synaptic transmission in aortic baroreceptor regions of nucleus trachus solitarius. J Neurophysiol. 1995;74:1518–1528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
