Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis
- PMID: 18045939
- PMCID: PMC2224448
- DOI: 10.1128/JVI.01600-07
Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis
Abstract
Hepatitis B and C viruses are major causative agents of liver fibrosis, cirrhosis, and liver cancer. Using comparative glycoproteomics, we identified a glycoprotein that is altered both in amount and in glycosylation as a function of liver fibrosis and cirrhosis. Specifically, this altered glycoprotein is an immunoglobulin G (IgG) molecule reactive to the heterophilic alpha-Gal epitope [Galalpha-1-3Galbeta1-(3)4GlcNAc-R]. While similar changes in glycosylation have been observed in several autoimmune diseases, the specific immunoglobulins and their antigen recognition profiles were not determined. Thus, we provide the first report identifying the specific antigenic recognition profile of an immunoglobulin molecule containing altered glycosylation as a function of liver disease. This change in glycosylation allowed increased reactivity with several fucose binding lectins and permitted the development of a plate-based assay to measure this change. Increased lectin reactivity was observed in 100% of the more than 200 individuals with stage III or greater fibrosis and appeared to be correlated with the degree of fibrosis. The reason for the alteration in the glycosylation of anti-Gal IgG is currently unclear but may be related to the natural history of the disease and may be useful in the noninvasive detection of fibrosis and cirrhosis.
Figures

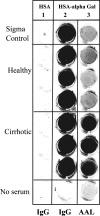
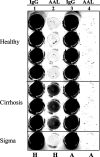




References
-
- Alter, M. J. 1997. Epidemiology of hepatitis C. Hepatology 2662S-65S. - PubMed
-
- Arnold, J. N., M. R. Wormald, R. B. Sim, P. M. Rudd, and R. A. Dwek. 2006. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2521-50. - PubMed
-
- Block, T. M., M. A. Comunale, M. Lowman, L. F. Steel, P. R. Romano, C. Fimmel, B. C. Tennant, W. T. London, A. A. Evans, B. S. Blumberg, R. A. Dwek, T. S. Mattu, and A. S. Mehta. 2005. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc. Natl. Acad. Sci. USA 102779-784. - PMC - PubMed
-
- Callewaert, N., H. Van Vlierberghe, A. Van Hecke, W. Laroy, J. Delanghe, and R. Contreras. 2004. Noninvasive diagnosis of liver cirrhosis using DNA sequencer based total serum protein glycomics. Nat. Med. 10429-434. - PubMed
-
- Comunale, M. A., M. Lowman, R. E. Long, J. Krakover, R. Philip, S. Seeholzer, A. A. Evans, H. W. L. Hann, T. M. Block, and A. S. Mehta. 2006. Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J. Proteome Res. 6308-315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

