Fibroblast adaptation and stiffness matching to soft elastic substrates
- PMID: 18045965
- PMCID: PMC2098710
- DOI: 10.1529/biophysj.106.101386
Fibroblast adaptation and stiffness matching to soft elastic substrates
Abstract
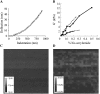
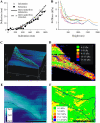
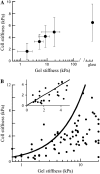
Many cell types alter their morphology and gene expression profile when grown on chemically equivalent surfaces with different rigidities. One expectation of this change in morphology and composition is that the cell's internal stiffness, governed by cytoskeletal assembly and production of internal stresses, will change as a function of substrate stiffness. Atomic force microscopy was used to measure the stiffness of fibroblasts grown on fibronectin-coated polyacrylamide gels of shear moduli varying between 500 and 40,000 Pa. Indentation measurements show that the cells' elastic moduli were equal to, or slightly lower than, those of their substrates for a range of soft gels and reached a saturating value at a substrate rigidity of 20 kPa. The amount of cross-linked F-actin sedimenting at low centrifugal force also increased with substrate stiffness. Together with enhanced actin polymerization and cross-linking, active contraction of the cytoskeleton can also modulate stiffness by exploiting the nonlinear elasticity of semiflexible biopolymer networks. These results suggest that within a range of stiffness spanning that of soft tissues, fibroblasts tune their internal stiffness to match that of their substrate, and modulation of cellular stiffness by the rigidity of the environment may be a mechanism used to direct cell migration and wound repair.
Figures


References
-
- Levental, I., P. C. Georges, and P. A. Janmey. 2007. Soft biological materials and their impact on cell function. Soft Matter. 1:299–306. - PubMed
-
- Engler, A. J., S. Sen, H. L. Sweeney, and D. E. Discher. 2006. Matrix elasticity directs stem cell lineage specification. Cell. 126:677–689. - PubMed
-
- Discher, D. E., P. Janmey, and Y. L. Wang. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science. 310:1139–1143. - PubMed
-
- Georges, P. C., and P. A. Janmey. 2005. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 98:1547–1553. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

