The severity of airways obstruction as a determinant of treatment response in COPD
- PMID: 18046858
- PMCID: PMC2707163
- DOI: 10.2147/copd.2006.1.3.209
The severity of airways obstruction as a determinant of treatment response in COPD
Abstract
Guidelines recommend that patients with COPD are stratified arbitrarily by baseline severity (FEV1) to decide when to initiate combination treatment with a long-acting beta2-agonist and an inhaled corticosteroid. Assessment of baseline FEV1 as a continuous variable may provide a more reliable prediction of treatment effects. Patients from a 1-year, parallel-group, randomized controlled trial comparing 50 microg salmeterol (Sal), 500 microg fluticasone propionate (FP), the combination (Sal/FP) and placebo, (bid), were categorized post hoc into FEV1 < 50% and FEV1 > or = 50% predicted subgroups (n = 949/513 respectively). Treatment effects on clinical outcomes-- lung function, exacerbations, health status, diary card symptoms, and adverse events--were investigated. Treatment responses based on a pre-specified analysis explored treatment differences by severity as a continuous variable. Lung function improved with active treatment irrespective of FEV1; Sal/FP had greatest effect. This improvement appeared additive in milder disease; synergistic in severe disease. Active therapy significantly reduced exacerbation rate in patients with FEV1 < 50% predicted, not in milder disease. Health status and breathlessness improved with Sal/FP irrespective of baseline FEV1; adverse events were similar across subgroups. The spirometric response to Sal/FP varied with baseline FEV1, and clinical benefits were not restricted to patients with severe disease. These data have implications for COPD management decisions, suggesting that arbitrary stratifications of baseline severity are not necessarily indicative of treatment efficacy and that the benefits of assessing baseline severity as a continuous variable should be assessed in future trials.
Figures

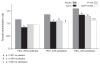

References
-
- American Thoracic Society/European Respiratory Society Task Force. Standards for the Diagnosis and Management of Patients with COPD [online] New York: American Thoracic Society; 2004. [Accessed 30 April 2006]. Version 1.2. URL: http://www.thoracic.org/copd/
-
- Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163:585–91. - PubMed
-
- [BTS] British Thoracic Society. British Thoracic Society COPD guidelines summary. Thorax. 1997;52(Suppl 5):1–32. - PubMed
-
- Brown H, Prescot R. Applied mixed models in medicine. In: Barnett V, editor. Repeated measures data. New York: J Wiley and Sons; 1999. pp. 199–259.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

