Doxazosin in the treatment of benign prostatic hypertrophy: an update
- PMID: 18046916
- PMCID: PMC2699642
- DOI: 10.2147/ciia.2006.1.4.389
Doxazosin in the treatment of benign prostatic hypertrophy: an update
Abstract
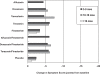
We evaluated the efficacy and safety of alpha 1--blocker doxazosin for treatment of lower urinary tract symptoms (LUTS) compatible with benign prostatic hypertrophy (BPH). Fourteen randomized controlled trials enrolled 6261 men, average age 64 years, who had moderately severe LUTS and flow impairment. Compared with baseline measures and placebo effect, doxazosin resulted in a statistically significant improvement in both LUTS and flow. However, when compared with placebo, the average magnitude of symptom improvement (International Prostate Symptom Score [IPSS] improvement < 3 points) typically did not achieve a level detectable by patients. Combined doxazosin and finasteride therapy improved LUTS and reduced the risk of overall clinical progression of BPH compared to each drug separately in men followed over 4 years. Reported mean changes from baseline in the IPSS were -7.4, -6.6, -5.6, and -4.9 points for combination therapy, doxazosin, finasteride, and placebo, respectively. Combination therapy reduced the need for invasive treatment for BPH and the risk of long-term urinary retention. The absolute reductions compared with placebo were less than 4% and primarily seen in men with prostate gland volume > 40 mL or PSA levels > 4 ng/mL. Efficacy was comparable with other alpha 1--blockers. Withdrawals from treatment for any cause were comparable to placebo. Dizziness and fatigue occurred more frequently with doxazosin compared to placebo.
Figures

References
-
- Akan H, Basar M, Dalva I, et al. The early effects of doxazosin on benign prostatic hyperplasia. Arch Ital Urol Androl. 1998;70:41–4. - PubMed
-
- Andersen M, Dahlstrand C, Hoye K. Double-blind trial of the efficacy and tolerability of doxazosin in the gastrointestinal therapeutic system, doxazosin standard, and placebo in patients with benign prostatic hyperplasia. Eur Urol. 2000;38:400–9. - PubMed
-
- [AUA] AUA Practice Guidelines Committee AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–47. - PubMed
-
- Barry MJ, Fowler FJ, Jr, Bin L, et al. A nationwide survey of practicing urologists: current management of benign prostatic hyperplasia and clinically localized prostate cancer. J Urol. 1997;158:488–91. - PubMed
-
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154:1770–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

