Patient preference in the management of postmenopausal osteoporosis with bisphosphonates
- PMID: 18046918
- PMCID: PMC2699650
- DOI: 10.2147/ciia.2006.1.4.415
Patient preference in the management of postmenopausal osteoporosis with bisphosphonates
Abstract
The leading treatments for postmenopausal osteoporosis are the nitrogen-containing bisphosphonates, which are required long term for optimal benefit. Oral bisphosphonates have proven efficacy in postmenopausal osteoporosis in clinical trials, but in practice the therapeutic benefits are often compromised by patients' low adherence. Nonadherence to bisphosphonate therapy negatively impacts outcomes such as fracture rate; fractures are in turn associated with decreased quality of life. The most common reason cited by patients for their nonadherence is that the strict dosing instructions for bisphosphonates are difficult to follow. One aspect of bisphosphonate administration that can be changed is dosing frequency and several studies have evaluated patient preferences for different dosing schedules. Studies have shown a preference for a weekly bisphosphonate regimen versus daily dosing and it has been demonstrated that this preference for reduced dosing frequency impacts on adherence. Ibandronate is the first nitrogen-containing oral bisphosphonate for osteoporosis that can be administered in a monthly regimen and two robust clinical studies demonstrated a strong patient preference for this monthly regimen versus a weekly regimen. It is important that physicians consider patient preference when prescribing treatment for osteoporosis to ensure that the disease is effectively managed for the long-term benefit of the patient.
Figures
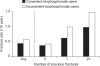
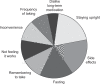


Similar articles
-
Clinical evaluation of novel bisphosphonate dosing regimens in osteoporosis: the role of comparative studies and implications for future studies.Clin Ther. 2007 Jun;29(6):1116-27. doi: 10.1016/j.clinthera.2007.06.009. Clin Ther. 2007. PMID: 17692726 Review.
-
Optimizing the management of postmenopausal osteoporosis with bisphosphonates: the emerging role of intermittent therapy.Clin Ther. 2005 Apr;27(4):361-76. doi: 10.1016/j.clinthera.2005.04.005. Clin Ther. 2005. PMID: 15922811 Review.
-
Patient preference and adherence: comparative US studies between two bisphosphonates, weekly risedronate and monthly ibandronate.Curr Med Res Opin. 2006 Dec;22(12):2383-91. doi: 10.1185/030079906X154042. Curr Med Res Opin. 2006. PMID: 17257452
-
Adherence, patient preference and dosing frequency: understanding the relationship.Bone. 2006 Apr;38(4 Suppl 1):S2-6. doi: 10.1016/j.bone.2006.01.150. Epub 2006 Mar 7. Bone. 2006. PMID: 16520104 Review.
-
Once-monthly oral ibandronate in postmenopausal osteoporosis: translation and updated review.Clin Ther. 2009 Jul;31(7):1497-510. doi: 10.1016/j.clinthera.2009.07.018. Clin Ther. 2009. PMID: 19695399 Review.
Cited by
-
Physicians' perspectives on the treatment of osteoporosis patients with bisphosphonates.Clin Interv Aging. 2016 Feb 15;11:1-8. doi: 10.2147/CIA.S97593. eCollection 2016. Clin Interv Aging. 2016. PMID: 26929609 Free PMC article.
-
Risk of renal impairment after treatment with ibandronate versus zoledronic acid: a retrospective medical records review.Support Care Cancer. 2009 Jun;17(6):719-25. doi: 10.1007/s00520-008-0553-7. Epub 2008 Dec 17. Support Care Cancer. 2009. PMID: 19089462
-
Efficacy, side effects and route of administration are more important than frequency of dosing of anti-osteoporosis treatments in determining patient adherence: a critical review of published articles from 1970 to 2009.Osteoporos Int. 2011 Mar;22(3):741-53. doi: 10.1007/s00198-010-1335-x. Epub 2010 Jun 30. Osteoporos Int. 2011. PMID: 20589368 Review.
-
[Cladribin. Development of an oral formulation for the treatment of multiple sclerosis].Nervenarzt. 2010 Feb;81(2):194-202. doi: 10.1007/s00115-009-2878-y. Nervenarzt. 2010. PMID: 20127230 Review. German.
-
Association Between Gastrointestinal Events and Health Care Resource Utilization Among Patients with Osteoporosis: Analysis of a U.S. Managed Care Population.J Manag Care Spec Pharm. 2015 Sep;21(9):811-21. doi: 10.18553/jmcp.2015.21.9.811. J Manag Care Spec Pharm. 2015. PMID: 26308228 Free PMC article.
References
-
- Baroutsou B, Babiolakis D, Stamatiadou A, et al. Patient compliance and preference of alendronate once weekly administration in comparison with daily regimens for osteoporotic postmenopausal women. Ann Rheum Dis. 2004;63(Suppl 1):455.
-
- Bartl R, Goette S, Hadji P, et al. Persistence and compliance with daily- and weekly-administered bisphosphonates in German women with osteoporosis. Ann Rheum Dis. 2006;64(Suppl 3):364.
-
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–41. - PubMed
-
- Brown JP, Kendler DL, McClung MR, et al. The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int. 2002;71:103–11. - PubMed
-
- Caro JJ, Ishak KJ, Huybrechts KF, et al. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

