Long-term stability of RNA in post-mortem bovine skeletal muscle, liver and subcutaneous adipose tissues
- PMID: 18047648
- PMCID: PMC2234424
- DOI: 10.1186/1471-2199-8-108
Long-term stability of RNA in post-mortem bovine skeletal muscle, liver and subcutaneous adipose tissues
Abstract
Background: Recovering high quality intact RNA from post-mortem tissue is of major concern for gene expression studies in animals and humans. Since the availability of post-mortem tissue is often associated with substantial delay, it is important that we understand the temporal variation in the stability of total RNA and of individual gene transcripts so as to be able to appropriately interpret the data generated from such studies. Hence, the objective of this experiment was to qualitatively and quantitatively assess the integrity of total and messenger RNA extracted from bovine skeletal muscle, subcutaneous adipose tissue and liver stored at 4 degrees C at a range of time points up to 22 days post-mortem. These conditions were designed to mimic the environment prevailing during the transport of beef from the abattoir to retail outlets.
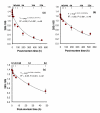
Results: The 28S and 18S rRNA molecules of total RNA were intact for up to 24 h post-mortem in liver and adipose tissues and up to 8 days post-mortem in skeletal muscle. The mRNA of housekeeping genes (GAPDH and ACTB) and two diet-related genes (RBP5 and SCD) were detectable up to 22 days post-mortem in skeletal muscle. While the mRNA stability of the two housekeeping genes was different in skeletal muscle and liver, they were similar to each other in adipose tissue. After 22 days post-mortem, the relative abundance of RBP5 gene was increased in skeletal muscle and in adipose tissue and decreased in liver. During this period, the relative abundance of SCD gene also increased in skeletal muscle whereas it decreased in both adipose tissue and liver.
Conclusion: Stability of RNA in three tissues (skeletal muscle, subcutaneous adipose tissue and liver) subjected to long-term post-mortem storage at refrigeration temperature indicated that skeletal muscle can be a suitable tissue for recovering biologically useful RNA for gene expression studies even if the tissue is subjected to post-mortem storage for weeks, whereas adipose tissue and liver should be processed within 24 hours post-mortem.
Figures



References
-
- Marchuk L, Sciore P, Reno C, Frank CB, Hart DA. Postmortem stability of total RNA isolated from rabbit ligament, tendon and cartilage. Biochim Biophys Acta. 1998;1379:171–177. - PubMed
-
- Fitzpatrick R, Casey OM, Morris D, Smith T, Powell R, Sreenan JM. Postmortem stability of RNA isolated from bovine reproductive tissues. Biochim Biophys Acta. 2002;1574:10–14. - PubMed
-
- Inoue H, Kimura A, Tuji T. Degradation profile of mRNA in a dead rat body: basic semi-quantification study. Forensic Sci Int. 2002;130:127–132. - PubMed
-
- Kuliwaba JS, Fazzalari NL, Findlay DM. Stability of RNA isolated from human trabecular bone at post-mortem and surgery. Biochim Biophys Acta. 2005;1740:1–11. - PubMed
-
- Seyboldt C, John A, Mueffling T, Nowak B, Wenzel S. Reverse transcription-polymerase chain reaction assay for species-specific detection of bovine central nervous system tissue in meat and meat products. J Food Prot. 2003;66:644–651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

