The role of the UPS in cystic fibrosis
- PMID: 18047735
- PMCID: PMC2106362
- DOI: 10.1186/1471-2091-8-S1-S11
The role of the UPS in cystic fibrosis
Abstract
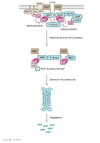
CF is an inherited autosomal recessive disease whose lethality arises from malfunction of CFTR, a single chloride (Cl-) ion channel protein. CF patients harbor mutations in the CFTR gene that lead to misfolding of the resulting CFTR protein, rendering it inactive and mislocalized. Hundreds of CF-related mutations have been identified, many of which abrogate CFTR folding in the endoplasmic reticulum (ER). More than 70% of patients harbor the DeltaF508 CFTR mutation that causes misfolding of the CFTR proteins. Consequently, mutant CFTR is unable to reach the apical plasma membrane of epithelial cells that line the lungs and gut, and is instead targeted for degradation by the UPS. Proteins located in both the cytoplasm and ER membrane are believed to identify misfolded CFTR for UPS-mediated degradation. The aberrantly folded CFTR protein then undergoes polyubiquitylation, carried out by an E1-E2-E3 ubiquitin ligase system, leading to degradation by the 26S proteasome. This ubiquitin-dependent loss of misfolded CFTR protein can be inhibited by the application of 'corrector' drugs that aid CFTR folding, shielding it from the UPS machinery. Corrector molecules elevate cellular CFTR protein levels by protecting the protein from degradation and aiding folding, promoting its maturation and localization to the apical plasma membrane. Combinatory application of corrector drugs with activator molecules that enhance CFTR Cl- ion channel activity offers significant potential for treatment of CF patients. Publication history: Republished from Current BioData's Targeted Proteins database (TPdb; http://www.targetedproteinsdb.com).
Figures


References
-
- Roomans G.M. Pharmacological approaches to correcting the ion transport defect in cystic fibrosis. Am J Respir Med. 2003;2:413–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

