Axial ligand tuning of a nonheme iron(IV)-oxo unit for hydrogen atom abstraction
- PMID: 18048327
- PMCID: PMC2148264
- DOI: 10.1073/pnas.0709471104
Axial ligand tuning of a nonheme iron(IV)-oxo unit for hydrogen atom abstraction
Abstract
The reactivities of mononuclear nonheme iron(IV)-oxo complexes bearing different axial ligands, [Fe(IV)(O)(TMC)(X)](n+) [where TMC is 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane and X is NCCH(3) (1-NCCH(3)), CF(3)COO(-) (1-OOCCF(3)), or N(3)(-) (1-N(3))], and [Fe(IV)(O)(TMCS)](+) (1'-SR) (where TMCS is 1-mercaptoethyl-4,8,11-trimethyl-1,4,8,11-tetraazacyclotetradecane), have been investigated with respect to oxo-transfer to PPh(3) and hydrogen atom abstraction from phenol O H and alkylaromatic C H bonds. These reactivities were significantly affected by the identity of the axial ligands, but the reactivity trends differed markedly. In the oxidation of PPh(3), the reactivity order of 1-NCCH(3) > 1-OOCCF(3) > 1-N(3) > 1'-SR was observed, reflecting a decrease in the electrophilicity of iron(IV)-oxo unit upon replacement of CH(3)CN with an anionic axial ligand. Surprisingly, the reactivity order was inverted in the oxidation of alkylaromatic C H and phenol O H bonds, i.e., 1'-SR > 1-N(3) > 1-OOCCF(3) > 1-NCCH(3). Furthermore, a good correlation was observed between the reactivities of iron(IV)-oxo species in H atom abstraction reactions and their reduction potentials, E(p,c), with the most reactive 1'-SR complex exhibiting the lowest potential. In other words, the more electron-donating the axial ligand is, the more reactive the iron(IV)-oxo species becomes in H atom abstraction. Quantum mechanical calculations show that a two-state reactivity model applies to this series of complexes, in which a triplet ground state and a nearby quintet excited-state both contribute to the reactivity of the complexes. The inverted reactivity order in H atom abstraction can be rationalized by a decreased triplet-quintet gap with the more electron-donating axial ligand, which increases the contribution of the much more reactive quintet state and enhances the overall reactivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

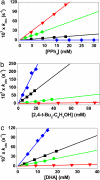

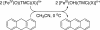
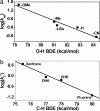
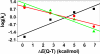
 H BDE of p-Y-2,6-t- Bu2C6H3OH in CH3CN at 25°C. (b) Plot of log k′2 of [FeIV(O)(TMC)(N3)]+ (1-N3) against C
H BDE of p-Y-2,6-t- Bu2C6H3OH in CH3CN at 25°C. (b) Plot of log k′2 of [FeIV(O)(TMC)(N3)]+ (1-N3) against C H BDE of substrates. Second-order rate constants, k2, were determined at 25°C and then adjusted for reaction stoichiometry to yield k′2 based on the number of equivalent target C
H BDE of substrates. Second-order rate constants, k2, were determined at 25°C and then adjusted for reaction stoichiometry to yield k′2 based on the number of equivalent target C H bonds of substrates (e.g., four for DHA and CHD and two for xanthene and fluorene).
H bonds of substrates (e.g., four for DHA and CHD and two for xanthene and fluorene).
Similar articles
-
Contrasting effects of axial ligands on electron-transfer versus proton-coupled electron-transfer reactions of nonheme oxoiron(IV) complexes.Chemistry. 2010 Jan 4;16(1):354-61. doi: 10.1002/chem.200901163. Chemistry. 2010. PMID: 19937616
-
Tuning reactivity and mechanism in oxidation reactions by mononuclear nonheme iron(IV)-oxo complexes.Acc Chem Res. 2014 Apr 15;47(4):1146-54. doi: 10.1021/ar400258p. Epub 2014 Feb 13. Acc Chem Res. 2014. PMID: 24524675
-
Synthetic mononuclear nonheme iron-oxygen intermediates.Acc Chem Res. 2015 Aug 18;48(8):2415-23. doi: 10.1021/acs.accounts.5b00218. Epub 2015 Jul 23. Acc Chem Res. 2015. PMID: 26203519
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
-
High-valent iron(IV)-oxo complexes of heme and non-heme ligands in oxygenation reactions.Acc Chem Res. 2007 Jul;40(7):522-31. doi: 10.1021/ar700027f. Epub 2007 May 1. Acc Chem Res. 2007. PMID: 17469792 Review.
Cited by
-
Singlet versus Triplet Reactivity in an Mn(V)-Oxo Species: Testing Theoretical Predictions Against Experimental Evidence.J Am Chem Soc. 2016 Sep 28;138(38):12375-86. doi: 10.1021/jacs.6b05027. Epub 2016 Sep 14. J Am Chem Soc. 2016. PMID: 27545752 Free PMC article.
-
C-H bond cleavage with reductants: re-investigating the reactivity of monomeric Mn(III/IV)-oxo complexes and the role of oxo ligand basicity.J Am Chem Soc. 2009 Mar 4;131(8):2762-3. doi: 10.1021/ja8100825. J Am Chem Soc. 2009. PMID: 19196005 Free PMC article.
-
Sulfur versus iron oxidation in an iron-thiolate model complex.J Am Chem Soc. 2010 Dec 8;132(48):17118-29. doi: 10.1021/ja1045428. Epub 2010 Nov 11. J Am Chem Soc. 2010. PMID: 21070030 Free PMC article.
-
Near-stoichiometric conversion of H(2)O(2) to Fe(IV)=O at a nonheme iron(II) center. Insights into the O-O bond cleavage step.J Am Chem Soc. 2010 Feb 24;132(7):2134-5. doi: 10.1021/ja9101908. J Am Chem Soc. 2010. PMID: 20121136 Free PMC article.
-
[Fe(IV)═O(TBC)(CH3CN)]2+: comparative reactivity of iron(IV)-oxo species with constrained equatorial cyclam ligation.J Am Chem Soc. 2012 Jul 18;134(28):11791-806. doi: 10.1021/ja3046298. Epub 2012 Jul 6. J Am Chem Soc. 2012. PMID: 22708532 Free PMC article.
References
-
- Nam W. Acc Chem Res. 2007;40:522–531. - PubMed
-
- Meunier B, de Visser SP, Shaik S. Chem Rev. 2004;104:3947–3980. - PubMed
-
- Groves JT. J Inorg Biochem. 2006;100:434–447. - PubMed
-
- Costas M, Mehn MP, Jensen MP, Que L., Jr Chem Rev. 2004;104:939–986. - PubMed
-
- Kryatov SV, Rybak-Akimova EV, Schindler S. Chem Rev. 2005;105:2175–2226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

