Inflammatory status influences aromatase and steroid receptor expression in endometriosis
- PMID: 18048499
- PMCID: PMC2275353
- DOI: 10.1210/en.2007-0665
Inflammatory status influences aromatase and steroid receptor expression in endometriosis
Abstract
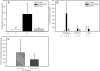
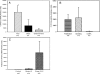
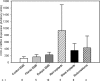
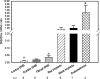
Aberrant up-regulation of aromatase in eutopic endometrium and implants from women with endometriosis has been reported. Aromatase induction may be mediated by increased cyclooxygenase-2 (COX-2). Recently, we demonstrated that progesterone receptor (PR)-A and PR-B serve an antiinflammatory role in the uterus by antagonizing nuclear factor kappaB activation and COX-2 expression. PR-C, which antagonizes PR-B, is up-regulated by inflammation. Although estrogen receptor alpha (ERalpha) is implicated in endometriosis, an antiinflammatory role of ERbeta has been suggested. We examined stage-specific expression of aromatase, COX-2, ER, and PR isoform expression in eutopic endometrium, implants, peritoneum, and endometrioma samples from endometriosis patients. Endometrial and peritoneal biopsies were obtained from unaffected women and those with fibroids. Aromatase expression in eutopic endometrium from endometriosis patients was significantly increased compared with controls. Aromatase expression in endometriosis implants was markedly increased compared with eutopic endometrium. Aromatase mRNA levels were increased significantly in red implants relative to black implants and endometrioma cyst capsule. Moreover, COX-2 expression was increased in implants and in eutopic endometrium of women with endometriosis as compared with control endometrium. As observed for aromatase mRNA, the highest levels of COX-2 mRNA were found in red implants. The ratio of ERbeta/ERalpha mRNA was significantly elevated in endometriomas compared with endometriosis implants and eutopic endometrium. Expression of PR-C mRNA relative to PR-A and PR-B mRNA was significantly increased in endometriomas compared with eutopic and control endometrium. PR-A protein was barely detectable in endometriomas. Thus, whereas PR-C may enhance disease progression, up-regulation of ERbeta may play an antiinflammatory and opposing role.
Figures




References
-
- Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, Fushiki S, Osawa Y, Honjo H 1997 Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod 57:514–519 - PubMed
-
- Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Zhou J, Sebastian S 2001 Role of aromatase in endometrial disease. J Steroid Biochem Mol Biol 79:19–25 - PubMed
-
- Yang S, Fang Z, Suzuki T, Sasano H, Zhou J, Gurates B, Tamura M, Ferrer K, Bulun S 2002 Regulation of aromatase P450 expression in endometriotic and endometrial stromal cells by CCAAT/enhancer binding proteins (C/EBPs): decreased C/EBPβ in endometriosis is associated with overexpression of aromatase. J Clin Endocrinol Metab 87:2336–2345 - PubMed
-
- Lebovic DI, Mueller MD, Taylor RN 2001 Immunobiology of endometriosis. Fertil Steril 75:1–10 - PubMed
-
- Lebovic DI, Chao VA, Martini JF, Taylor RN 2001 IL-1β induction of RANTES (regulated upon activation, normal T cell expressed and secreted) chemokine gene expression in endometriotic stromal cells depends on a nuclear factor-κB site in the proximal promoter. J Clin Endocrinol Metab 86:4759–4764 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

