ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia
- PMID: 18048647
- PMCID: PMC2254551
- DOI: 10.1182/blood-2006-12-062265
ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia
Abstract
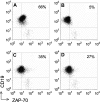
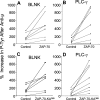
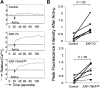
We transduced chronic lymphocytic leukemia (CLL) cells lacking ZAP-70 with vectors encoding ZAP-70 or various mutant forms of ZAP-70 and monitored the response of transduced CLL cells to treatment with F(ab)(2) anti-IgM (anti-mu). CLL cells made to express ZAP-70, a kinase-defective ZAP-70 (ZAP-70-KA(369)), or a ZAP-70 unable to bind c-Cbl (ZAP-YF(292)) experienced greater intracellular calcium flux and had greater increases in the levels of phosphorylated p72(Syk), B-cell linker protein (BLNK), and phospholipase C-gamma, and greater activation of the Ig accessory molecule CD79b in response to treatment with anti-mu than did mock-transfected CLL cells lacking ZAP-70. Transfection of CLL cells with vectors encoding truncated forms of ZAP-70 revealed that the SH2 domain, but not the SH1 domain, was necessary to enhance intracellular calcium flux in response to treatment with anti-mu. We conclude that ZAP-70 most likely acts as an adapter protein that facilitates B-cell receptor (BCR) signaling in CLL cells independent of its tyrosine kinase activity or its ability to interact with c-Cbl.
Figures



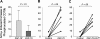
References
-
- Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. - PubMed
-
- Qian D, Weiss A. T cell antigen receptor signal transduction. Curr Opin Cell Biol. 1997;9:205–212. - PubMed
-
- Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12:242–249. - PubMed
-
- Chan AC, van Oers NS, Tran A, et al. Differential expression of ZAP-70 and Syk protein tyrosine kinases, and the role of this family of protein tyrosine kinases in TCR signaling. J Immunol. 1994;152:4758–4766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

