Interferons at age 50: past, current and future impact on biomedicine
- PMID: 18049472
- PMCID: PMC7097588
- DOI: 10.1038/nrd2422
Interferons at age 50: past, current and future impact on biomedicine
Abstract
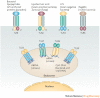
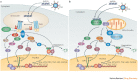
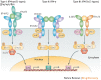
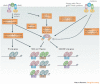
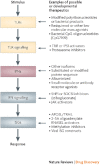
The family of interferon (IFN) proteins has now more than reached the potential envisioned by early discovering virologists: IFNs are not only antivirals with a spectrum of clinical effectiveness against both RNA and DNA viruses, but are also the prototypic biological response modifiers for oncology, and show effectiveness in suppressing manifestations of multiple sclerosis. Studies of IFNs have resulted in fundamental insights into cellular signalling mechanisms, gene transcription and innate and acquired immunity. Further elucidation of the multitude of IFN-induced genes, as well as drug development strategies targeting IFN production via the activation of the Toll-like receptors (TLRs), will almost certainly lead to newer and more efficacious therapeutics. Our goal is to offer a molecular and clinical perspective that will enable IFNs or their TLR agonist inducers to reach their full clinical potential.
Conflict of interest statement
The authors declare that they have no competing interests that constitute a conflict of interest for content and interpretations contained herein with the following exceptions: E.C.B. is a member of the Scientific Advisory Boards of Cleveland BioLabs and Alios BioPharma and in the past 3 years that of Coley Pharma together with honoaria for scientfic or educational presentations underwritten by Schering Plough, Maxygen, and Novartis; R.H.S. is a member of the Scientific Advisory Board of Alios BioPharma, Inc.; G.R.F. has lectured and consulted for Roche, Maxygen, Novartis, and Human Genome Sciences. Within the past 5 years, R.M.R. has received occasional honouraria for scientific presentations from (in alphabetical order) Berlex, Biogen and Serono. These presentations did not serve marketing interests.
Figures

References
-
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc. R. Soc. Lond., B, Biol. Sci. 1957;147:258–267. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Sen GC, Sarkar SN. Transcriptional signaling by double-stranded RNA: role of TLR3. Cytokine Growth Factor Rev. 2005;16:1–14. - PubMed
-
- Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunol. 2004;5:730–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA090914/CA/NCI NIH HHS/United States
- R01 CA103943/CA/NCI NIH HHS/United States
- CA089132/CA/NCI NIH HHS/United States
- CA115494/CA/NCI NIH HHS/United States
- CA68782/CA/NCI NIH HHS/United States
- P01 NS038667/NS/NINDS NIH HHS/United States
- NS32151/NS/NINDS NIH HHS/United States
- R01 NS032151/NS/NINDS NIH HHS/United States
- R01 CA044059/CA/NCI NIH HHS/United States
- R01 CA089132/CA/NCI NIH HHS/United States
- CA62220/CA/NCI NIH HHS/United States
- P01 CA062220/CA/NCI NIH HHS/United States
- R01 CA068782/CA/NCI NIH HHS/United States
- CA103943/CA/NCI NIH HHS/United States
- CA044059/CA/NCI NIH HHS/United States
- M01 RR018390/RR/NCRR NIH HHS/United States
- P50 NS038667/NS/NINDS NIH HHS/United States
- R01 CA90914/CA/NCI NIH HHS/United States
- NS38667/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

