Synthesis and characterization of ruthenium bis(beta-diketonato) pyridine-imidazole complexes for hydrogen atom transfer
- PMID: 18052056
- PMCID: PMC2596074
- DOI: 10.1021/ic7015726
Synthesis and characterization of ruthenium bis(beta-diketonato) pyridine-imidazole complexes for hydrogen atom transfer
Abstract
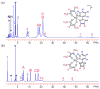
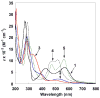
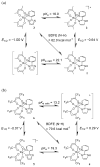
Ruthenium bis(beta-diketonato) complexes have been prepared at both the RuII and RuIII oxidation levels and with protonated and deprotonated pyridine-imidazole ligands. RuII(acac)2(py-imH) (1), [RuIII(acac)2(py-imH)]OTf (2), RuIII(acac)2(py-im) (3), RuII(hfac)2(py-imH) (4), and [DBU-H][RuII(hfac)2(py-im)] (5) have been fully characterized, including X-ray crystal structures (acac = 2,4-pentanedionato, hfac = 1,1,1,5,5,5-hexafluoro-2,4-pentanedionato, py-imH = 2-(2'-pyridyl)imidazole, DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene). For the acac-imidazole complexes 1 and 2, cyclic voltammetry in MeCN shows the RuIII/II reduction potential (E1/2) to be -0.64 V versus Cp2Fe+/0. E1/2 for the deprotonated imidazolate complex 3 (-1.00 V) is 0.36 V more negative. The RuII bis-hfac analogues 4 and 5 show the same DeltaE1/2 = 0.36 V but are 0.93 V harder to oxidize than the acac derivatives (0.29 and -0.07 V). The difference in acidity between the acac and hfac derivatives is much smaller, with pKa values of 22.1 and 19.3 in MeCN for 1 and 4, respectively. From the E1/2 and pKa values, the bond dissociation free energies (BDFEs) of the N-H bonds in 1 and 4 are calculated to be 62.0 and 79.6 kcal mol(-1) in MeCN - a remarkable difference of 17.6 kcal mol(-1) for such structurally similar compounds. Consistent with these values, there is a facile net hydrogen atom transfer from 1 to TEMPO* (2,2,6,6-tetramethylpiperidine-1-oxyl radical) to give 3 and TEMPO-H. The DeltaG degrees for this reaction is -4.5 kcal mol(-1). 4 is not oxidized by TEMPO* (DeltaG degrees = +13.1 kcal mol(-1)), but in the reverse direction TEMPO-H readily reduces in situ generated RuIII(hfac)2(py-im) (6). A RuII-imidazoline analogue of 1, RuII(acac)2(py-imnH) (7), reacts with 3 equiv of TEMPO* to give the imidazolate 3 and TEMPO-H, with dehydrogenation of the imidazoline ring.
Figures
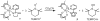
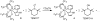
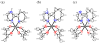
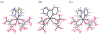

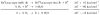
Similar articles
-
Hydrogen atom transfer reactions of a ruthenium imidazole complex: hydrogen tunneling and the applicability of the Marcus cross relation.J Am Chem Soc. 2008 Nov 5;130(44):14745-54. doi: 10.1021/ja805067h. Epub 2008 Oct 9. J Am Chem Soc. 2008. PMID: 18841973 Free PMC article.
-
Trends in ground-state entropies for transition metal based hydrogen atom transfer reactions.J Am Chem Soc. 2009 Apr 1;131(12):4335-45. doi: 10.1021/ja8081846. J Am Chem Soc. 2009. PMID: 19275235 Free PMC article.
-
Diorganoruthenium complexes incorporating noninnocent [C(6)H(2)(CH(2)ER(2))(2)-3,5](2)(2-) (E = N, P) bis-pincer bridging ligands: synthesis, spectroelectrochemistry, and DFT studies.Inorg Chem. 2007 Dec 24;46(26):11133-44. doi: 10.1021/ic701501q. Epub 2007 Dec 1. Inorg Chem. 2007. PMID: 18052055
-
Facile concerted proton-electron transfers in a ruthenium terpyridine-4'-carboxylate complex with a long distance between the redox and basic sites.J Am Chem Soc. 2008 Jun 11;130(23):7210-1. doi: 10.1021/ja801672w. Epub 2008 May 14. J Am Chem Soc. 2008. PMID: 18479096 Free PMC article.
-
Effects of trans-pyridine ligands on the interactions of RuII and RuIII ammine complexes N7-coordinated to purine nucleosides and DNA.J Biol Inorg Chem. 1999 Jun;4(3):325-40. doi: 10.1007/s007750050319. J Biol Inorg Chem. 1999. PMID: 10439078
Cited by
-
A Continuum of Proton-Coupled Electron Transfer Reactivity.Acc Chem Res. 2018 Oct 16;51(10):2391-2399. doi: 10.1021/acs.accounts.8b00319. Epub 2018 Sep 20. Acc Chem Res. 2018. PMID: 30234963 Free PMC article.
-
Structural, Electronic and Thermochemical preference for multi-PCET reactivity of Ruthenium(II)-Amine and Ruthenium(IV)-Amido Complexes.Eur J Inorg Chem. 2021 Oct 21;2021(39):4042. doi: 10.1002/ejic.202100761. Epub 2021 Sep 12. Eur J Inorg Chem. 2021. PMID: 34776777 Free PMC article.
-
Sm(II)-Mediated Proton-Coupled Electron Transfer: Quantifying Very Weak N-H and O-H Homolytic Bond Strengths and Factors Controlling Them.J Am Chem Soc. 2022 Nov 23;144(46):21337-21346. doi: 10.1021/jacs.2c09580. Epub 2022 Nov 8. J Am Chem Soc. 2022. PMID: 36346706 Free PMC article.
-
Hydrogen atom transfer reactions of a ruthenium imidazole complex: hydrogen tunneling and the applicability of the Marcus cross relation.J Am Chem Soc. 2008 Nov 5;130(44):14745-54. doi: 10.1021/ja805067h. Epub 2008 Oct 9. J Am Chem Soc. 2008. PMID: 18841973 Free PMC article.
-
Reversible Homolysis of a Carbon-Carbon σ-Bond Enabled by Complexation-Induced Bond-Weakening.J Am Chem Soc. 2022 Aug 31;144(34):15488-15496. doi: 10.1021/jacs.2c01229. Epub 2022 Aug 22. J Am Chem Soc. 2022. PMID: 35994332 Free PMC article.
References
-
- Cukier RI, Nocera DG. Annu Rev Phys Chem. 1998;49:337. - PubMed
- Hodgkiss JM, Rosenthal J, Nocera DG. In: Hydrogen-Transfer Reactions. Hynes JT, Klinman JP, Limbach H-H, Schowen RL, editors. Vol. 2. Wiley-VCH; Weinheim: 2007. pp. 503–562.
- Stubbe J, Nocera DG, Yee CS, Chang MCY. Chem Rev. 2003;103:2167. - PubMed
- Hammes-Schiffer S. In: Hydrogen-Transfer Reactions. Hynes JT, Klinman JP, Limbach H-H, Schowen RL, editors. Vol. 2. Wiley-VCH; Weinheim: 2007. pp. 479–502.
- Meyer TJ, Huynh MHV. Inorg Chem. 2003;42:8140. - PubMed
-
- Mayer JM. Annu Rev Phys Chem. 2004;55:363. - PubMed
- Mayer JM, Rhile IJ. Biochim Biophys Acta. 2004;1655:51. - PubMed
- Mayer JM, Rhile IJ, Larsen FB, Mader EA, Markle TF, DiPasquale AG. Photosynth Res. 2006;87:3. - PubMed
- Mayer JM, Mader EA, Roth JP, Bryant JR, Matsuo T, Dehestani A, Bales BC, Watson EJ, Osako T, Valliant-Saunders K, Lam W-H, Hrovat DA, Borden WT, Davidson ER. J Mol Catal A: Chem. 2006;251:24.
-
- Mayer JM. Acc Chem Res. 1998;31:441.
-
- Knapp MJ, Meyer M, Klinman JP. In: Hydrogen-Transfer Reactions. Hynes JT, Klinman JP, Limbach H-H, Schowen RL, editors. Vol. 4. Wiley-VCH; Weinheim: 2007. pp. 1241–1284.
- Knapp MJ, Rickert K, Klinman JP. J Am Chem Soc. 2002;124:3865. - PubMed
- Glickman MH, Klinman JP. Biochemistry. 1996;35:12882. - PubMed
-
-
There are also many examples of HAT involving metal hydride complexes, for instance: Song JS, Bullock RM, Creutz C. J Am Chem Soc. 1991;113:9862.Edidin RT, Sullivan JM, Norton JR. J Am Chem Soc. 1987;109:3945.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

