Disposition of flavonoids via enteric recycling: enzyme stability affects characterization of prunetin glucuronidation across species, organs, and UGT isoforms
- PMID: 18052087
- PMCID: PMC2532587
- DOI: 10.1021/mp700135a
Disposition of flavonoids via enteric recycling: enzyme stability affects characterization of prunetin glucuronidation across species, organs, and UGT isoforms
Abstract
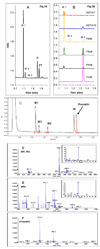
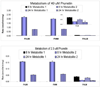
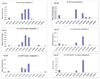
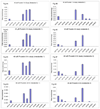
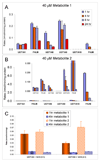
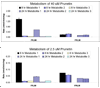
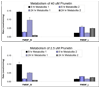
We characterized the in vitro glucuronidation of prunetin, a prodrug of genistein that is a highly active cancer prevention agent. Metabolism studies were conducted using expressed human UGT isoforms and microsomes/S9 fractions prepared from intestine and liver of rodents and humans. The results indicated that human intestinal microsomes were more efficient than liver microsomes in glucuronidating prunetin, but rates of metabolism were dependent on time of incubation at 37 degrees C. Human liver and intestinal microsomes mainly produced metabolite 1 (prunetin-5- O-glucuronide) and metabolite 2 (prunetin-4'- O-glucuronide), respectively. Using 12 human UGT isoforms, we showed that UGT1A7, UGT1A8, and UGT1A9 were mainly responsible for the formation of metabolite 1, whereas UGT1A1, UGT1A8, and UGT1A10 were mainly responsible for the formation of metabolite 2. This isoform-specific metabolism was consistent with earlier results obtained using human liver and intestinal microsomes, as the former (liver) is UGT1A9-rich whereas the latter is UGT1A10-rich. Surprisingly, we found that the thermostability of the microsomes was isoform- and organ-dependent. For example, human liver microsomal UGT activities were much more heat-stable (37 degrees C) than intestinal microsomal UGT activities, consistent with the finding that human UGT1A9 is much more thermostable than human UGT1A10 and UGT1A8. The organ-specific thermostability profiles were also evident in rat microsomes and mouse S9 fractions, even though human intestinal glucuronidation of prunetin differs significantly from rodent intestinal glucuronidation. In conclusion, prunetin glucuronidation is species-, organ-, and UGT-isoform-dependent, all of which may be impacted by the thermostability of specific UGT isoforms involved in the metabolism.
Figures

Similar articles
-
Structure and concentration changes affect characterization of UGT isoform-specific metabolism of isoflavones.Mol Pharm. 2009 Sep-Oct;6(5):1466-82. doi: 10.1021/mp8002557. Mol Pharm. 2009. PMID: 19545173 Free PMC article.
-
Disposition of flavonoids via enteric recycling: UDP-glucuronosyltransferase (UGT) 1As deficiency in Gunn rats is compensated by increases in UGT2Bs activities.J Pharmacol Exp Ther. 2009 Jun;329(3):1023-31. doi: 10.1124/jpet.108.147371. Epub 2009 Mar 5. J Pharmacol Exp Ther. 2009. PMID: 19264971 Free PMC article.
-
Troglitazone glucuronidation in human liver and intestine microsomes: high catalytic activity of UGT1A8 and UGT1A10.Drug Metab Dispos. 2002 Dec;30(12):1462-9. doi: 10.1124/dmd.30.12.1462. Drug Metab Dispos. 2002. PMID: 12433820
-
Identification of UDP-glucuronosyltransferases responsible for the glucuronidation of darexaban, an oral factor Xa inhibitor, in human liver and intestine.Drug Metab Dispos. 2012 Feb;40(2):276-82. doi: 10.1124/dmd.111.042614. Epub 2011 Oct 26. Drug Metab Dispos. 2012. PMID: 22031623
-
Characterization of benzo(a)pyrene-trans-7,8-dihydrodiol glucuronidation by human tissue microsomes and overexpressed UDP-glucuronosyltransferase enzymes.Cancer Res. 2002 Apr 1;62(7):1978-86. Cancer Res. 2002. PMID: 11929814
Cited by
-
Three-dimensional quantitative structure-activity relationship studies on UGT1A9-mediated 3-O-glucuronidation of natural flavonols using a pharmacophore-based comparative molecular field analysis model.J Pharmacol Exp Ther. 2011 Feb;336(2):403-13. doi: 10.1124/jpet.110.175356. Epub 2010 Nov 10. J Pharmacol Exp Ther. 2011. PMID: 21068207 Free PMC article.
-
Evaluation of 3,3',4'-trihydroxyflavone and 3,6,4'-trihydroxyflavone (4'-O-glucuronidation) as the in vitro functional markers for hepatic UGT1A1.Mol Pharm. 2011 Dec 5;8(6):2379-89. doi: 10.1021/mp200300w. Epub 2011 Oct 21. Mol Pharm. 2011. PMID: 21985641 Free PMC article.
-
Use of isoform-specific UGT metabolism to determine and describe rates and profiles of glucuronidation of wogonin and oroxylin A by human liver and intestinal microsomes.Pharm Res. 2010 Aug;27(8):1568-83. doi: 10.1007/s11095-010-0148-0. Epub 2010 Apr 22. Pharm Res. 2010. PMID: 20411407 Free PMC article.
-
Regioselective sulfation and glucuronidation of phenolics: insights into the structural basis.Curr Drug Metab. 2011 Nov;12(9):900-16. doi: 10.2174/138920011797470100. Curr Drug Metab. 2011. PMID: 21933112 Free PMC article. Review.
-
A Potential Alternative against Neurodegenerative Diseases: Phytodrugs.Oxid Med Cell Longev. 2016;2016:8378613. doi: 10.1155/2016/8378613. Epub 2016 Jan 6. Oxid Med Cell Longev. 2016. PMID: 26881043 Free PMC article. Review.
References
-
- Ando Y, Hasegawa Y. Clinical pharmacogenetics of irinotecan (CPT-11) Drug Metab Rev. 2005;37(3):565–574. - PubMed
-
- Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos. 2000;28(3):298–307. - PubMed
-
- Malfatti MA, Felton JS. Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of N-hydroxy-PhIP in vitro. Chem Res Toxicol. 2004;17(8):1137–1144. - PubMed
-
- Krishnaswamy S, Duan SX, Von Moltke LL, Greenblatt DJ, Sudmeier JL, Bachovchin WW, Court MH. Serotonin (5-hydroxytryptamine) glucuronidation in vitro: assay development, human liver microsome activities and species differences. Xenobiotica. 2003;33(2):169–180. - PubMed
-
- Yamanaka H, Nakajima M, Katoh M, Kanoh A, Tamura O, Ishibashi H, Yokoi T. Trans-3'-hydroxycotinine O- and N-glucuronidations in human liver microsomes. Drug Metab Dispos. 2005;33(1):23–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

