Potential mechanisms of estrogen quinone carcinogenesis
- PMID: 18052105
- PMCID: PMC2556295
- DOI: 10.1021/tx700191p
Potential mechanisms of estrogen quinone carcinogenesis
Abstract
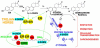
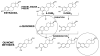
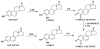
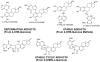
There is a clear association between the excessive exposure to estrogens and the development of cancer in hormone-sensitive tissues (breast, endometrium). It has become clear that there are likely multiple overlapping mechanisms of estrogen carcinogenesis. One major pathway is the extensively studied hormonal pathway, by which estrogen stimulates cell proliferation through nuclear estrogen receptor (ER)-mediated signaling, thus resulting in an increased risk of genomic mutations during DNA replication. A similar "nongenomic pathway", potentially involving newly discovered membrane-associated ERs, also appears to regulate extranuclear estrogen signaling pathways. This perspective is focused on a third pathway involving the metabolism of estrogens to catechols mediated by cytochrome P450 and further oxidation of these catechols to estrogen o-quinones. Oxidative enzymes, metal ions, and in some cases molecular oxygen can catalyze o-quinone formation, so that these electrophilic/redox-active quinones can cause damage within cells by alkylation and/or oxidation of cellular proteins and DNA in many tissues. It appears that the endogenous estrogen quinones primarily form unstable N3-adenine or N7-guanine DNA adducts, ultimately resulting in mutagenic apurinic sites. In contrast, equine estrogen quinones, formed from estrogens present in popular hormone replacement therapy prescriptions, generate a variety of DNA lesions, including bulky stable adducts, apurinic sites, DNA strand cleavage, and oxidation of DNA bases. DNA damage induced by these equine quinones is significantly increased in cells containing ERs, leading us to hypothesize a mechanism involving ER binding/alkylation by the catchol/quinone, resulting in a "Trojan horse". The "Trojan horse" carries the highly redox-active catechol to estrogen -sensitive genes, where high amounts of reactive oxygen species are generated, causing selective DNA damage. Our data further suggest that other key protein targets for estrogen o-quinones could be redox-sensitive enzymes (i.e, GST P1-1, QR). These proteins are involved in stress response cascades that are known to contribute to the regulation of cell proliferation and apoptosis. Finally, it has been shown that catechol estrogens can transform breast epithelial cells into a tumorigenic phenotype and that these transformed cells had differential gene expression of several genes involved in oxidative stress. Given the direct link between excessive exposure to estrogens, metabolism of estrogens, and increased risk of breast cancer, it is crucial that factors that affect the formation, reactivity, and cellular targets of estrogen quinoids be thoroughly explored.
Figures
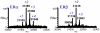
References
-
- Liehr JG. Genotoxic effects of estrogens. Mutation Res. 1990;238:269–276. - PubMed
-
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006;354:270–282. - PubMed
-
- Colditz GA. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J. Natl. Cancer Inst. 1998;90:814–823. - PubMed
-
- Feigelson HS, Henderson BE. Estrogens and breast cancer. Carcinogenesis. 1996;17:2279–2284. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288:321–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

