Crystal structure of a type II dihydrofolate reductase catalytic ternary complex
- PMID: 18052202
- PMCID: PMC3743094
- DOI: 10.1021/bi701532r
Crystal structure of a type II dihydrofolate reductase catalytic ternary complex
Abstract
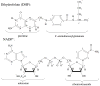
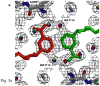
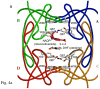
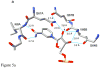
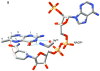
Type II dihydrofolate reductase (DHFR) is a plasmid-encoded enzyme that confers resistance to bacterial DHFR-targeted antifolate drugs. It forms a symmetric homotetramer with a central pore which functions as the active site. Its unusual structure, which results in a promiscuous binding surface that accommodates either the dihydrofolate (DHF) substrate or the NADPH cofactor, has constituted a significant limitation to efforts to understand its substrate specificity and reaction mechanism. We describe here the first structure of a ternary R67 DHFR.DHF.NADP+ catalytic complex, resolved to 1.26 A. This structure provides the first clear picture of how this enzyme, which lacks the active site carboxyl residue that is ubiquitous in Type I DHFRs, is able to function. In the catalytic complex, the polar backbone atoms of two symmetry-related I68 residues provide recognition motifs that interact with the carboxamide on the nicotinamide ring, and the N3-O4 amide function on the pteridine ring. This set of interactions orients the aromatic rings of substrate and cofactor in a relative endo geometry in which the reactive centers are held in close proximity. Additionally, a central, hydrogen-bonded network consisting of two pairs of Y69-Q67-Q67'-Y69' residues provides an unusually tight interface, which appears to serve as a "molecular clamp" holding the substrates in place in an orientation conducive to hydride transfer. In addition to providing the first clear insight regarding how this extremely unusual enzyme is able to function, the structure of the ternary complex provides general insights into how a mutationally challenged enzyme, i.e., an enzyme whose evolution is restricted to four-residues-at-a-time active site mutations, overcomes this fundamental limitation.
Figures







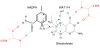
References
-
- Pattishall KH, Acar J, Burchall JJ, Goldstein FW, Harvey RJ. Two distinct types of trimethoprim-resistant dihydrofolate reductase specified by R-plasmids of different compatibility groups. J Biol Chem. 1977;252:2319–23. - PubMed
-
- Stone D, Smith SL. The amino acid sequence of the trimethoprim- resistant dihydrofolate reductase specified in Escherichia coli by R-plasmid R67. J Biol Chem. 1979;254:10857–61. - PubMed
-
- Smith SL, Stone D, Novak P, Baccanari DP, Burchall JJ. R plasmid dihydrofolate reductase with subunit structure. J Biol Chem. 1979;254:6222–5. - PubMed
-
- Narayana N, Matthews DA, Howell EE, Nguyen-huu X. A plasmid-encoded dihydrofolate reductase from trimethoprim-resistant bacteria has a novel D2-symmetric active site. Nat Struct Biol. 1995;2:1018–1025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

