Low-temperature pulsed EPR study at 34 GHz of the triplet states of the primary electron Donor P865 and the carotenoid in native and mutant bacterial reaction centers of Rhodobacter sphaeroides
- PMID: 18052205
- PMCID: PMC2562510
- DOI: 10.1021/bi701593r
Low-temperature pulsed EPR study at 34 GHz of the triplet states of the primary electron Donor P865 and the carotenoid in native and mutant bacterial reaction centers of Rhodobacter sphaeroides
Abstract
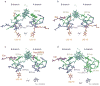
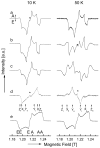
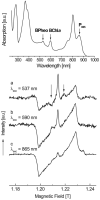
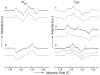
The photosynthetic charge separation in bacterial reaction centers occurs predominantly along one of two nearly symmetric branches of cofactors. Low-temperature EPR spectra of the triplet states of the chlorophyll and carotenoid pigments in the reaction center of Rhodobacter sphaeroides R-26.1, 2.4.1 and two double-mutants GD(M203)/AW(M260) and LH(M214)/AW(M260) have been recorded at 34 GHz to investigate the relative activities of the "A" and "B" branches. The triplet states are found to derive from radical pair and intersystem crossing mechanisms, and the rates of formation are anisotropic. The former mechanism is operative for Rb. sphaeroides R-26.1, 2.4.1, and mutant GD(M203)/AW(M260) and indicates that A-branch charge separation proceeds at temperatures down to 10 K. The latter mechanism, derived from the spin polarization and operative for mutant LH(M214)/AW(M260), indicates that no long-lived radical pairs are formed upon direct excitation of the primary donor and that virtually no charge separation at the B-branch occurs at low temperatures. When the temperature is raised above 30 K, B-branch charge separation is observed, which is at most 1% of A-branch charge separation. B-branch radical pair formation can be induced at 10 K with low yield by direct excitation of the bacteriopheophytin of the B-branch at 590 nm. The formation of a carotenoid triplet state is observed. The rate of formation depends on the orientation of the reaction center in the magnetic field and is caused by a magnetic field dependence of the oscillation frequency by which the singlet and triplet radical pair precursor states interchange. Combination of these findings with literature data provides strong evidence that the thermally activated transfer step on the B-branch occurs between the primary donor, P865, and the accessory bacteriochlorophyll, whereas this step is barrierless down to 10 K along the A-branch.
Figures





Similar articles
-
Bacteriopheophytin triplet state in Rhodobacter sphaeroides reaction centers.Photosynth Res. 2016 Aug;129(2):205-16. doi: 10.1007/s11120-016-0290-6. Epub 2016 Jul 1. Photosynth Res. 2016. PMID: 27368166 Free PMC article.
-
B-branch electron transfer in the photosynthetic reaction center of a Rhodobacter sphaeroides quadruple mutant. Q- and W-band electron paramagnetic resonance studies of triplet and radical-pair cofactor states.J Phys Chem B. 2010 Nov 18;114(45):14364-72. doi: 10.1021/jp1003424. Epub 2010 Mar 26. J Phys Chem B. 2010. PMID: 20345158
-
Triplet energy transfer between the primary donor and carotenoids in Rhodobacter sphaeroides R-26.1 reaction centers incorporated with spheroidene analogs having different extents of pi-electron conjugation.Photochem Photobiol. 1997 Jul;66(1):97-104. doi: 10.1111/j.1751-1097.1997.tb03144.x. Photochem Photobiol. 1997. PMID: 9230708
-
High yield of B-branch electron transfer in a quadruple reaction center mutant of the photosynthetic bacterium Rhodobacter sphaeroides.Biochemistry. 2002 Mar 5;41(9):3081-8. doi: 10.1021/bi011450m. Biochemistry. 2002. PMID: 11863447
-
Triplet state energy transfer between the primary donor and the carotenoid in Rhodobacter sphaeroides R-26.1 reaction centers exchanged with modified bacteriochlorophyll pigments and reconstituted with spheroidene.Photochem Photobiol. 1996 Nov;64(5):823-31. doi: 10.1111/j.1751-1097.1996.tb01842.x. Photochem Photobiol. 1996. PMID: 8931381
Cited by
-
Bacteriopheophytin triplet state in Rhodobacter sphaeroides reaction centers.Photosynth Res. 2016 Aug;129(2):205-16. doi: 10.1007/s11120-016-0290-6. Epub 2016 Jul 1. Photosynth Res. 2016. PMID: 27368166 Free PMC article.
-
Charge stabilization in reaction center protein investigated by optical heterodyne detected transient grating spectroscopy.Eur Biophys J. 2008 Sep;37(7):1167-74. doi: 10.1007/s00249-008-0294-z. Epub 2008 Mar 11. Eur Biophys J. 2008. PMID: 18330555
-
Comparative ENDOR study at 34 GHz of the triplet state of the primary donor in bacterial reaction centers of Rb. sphaeroides and Bl. viridis.Photosynth Res. 2014 May;120(1-2):99-111. doi: 10.1007/s11120-012-9786-x. Epub 2012 Nov 25. Photosynth Res. 2014. PMID: 23184403
References
-
- Blankenship B, Madigan M, Bauer C. Anoxygenic Photosynthetic Bacteria. Kluwer Academic Publishers; 1995.
-
- Thurnauer MC. ESR study of the photoexcited triplet state in photosynthetic bacteria. Rev Chem Int. 1979;100:197–231.
-
- Angerhofer A. Chlorophyll triplets and radical pairs. In: Scheer H, editor. Chlorophylls. CRC Press; Boca Raton FL: 1991. pp. 945–991.
-
- Feher G, Allen JP, Okamura MY, Rees DC. Structure and Function of Bacterial Photosynthetic Reaction Centers. Nature. 1989;339:111–116.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

