Caught in the Act: the 1.5 A resolution crystal structures of the HIV-1 protease and the I54V mutant reveal a tetrahedral reaction intermediate
- PMID: 18052235
- PMCID: PMC2546526
- DOI: 10.1021/bi700822g
Caught in the Act: the 1.5 A resolution crystal structures of the HIV-1 protease and the I54V mutant reveal a tetrahedral reaction intermediate
Abstract
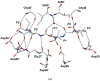
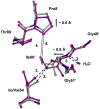
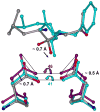
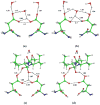
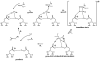
HIV-1 protease (PR) is the target for several important antiviral drugs used in AIDS therapy. The drugs bind inside the active site cavity of PR where normally the viral polyprotein substrate is bound and hydrolyzed. We report two high-resolution crystal structures of wild-type PR (PRWT) and the multi-drug-resistant variant with the I54V mutation (PRI54V) in complex with a peptide at 1.46 and 1.50 A resolution, respectively. The peptide forms a gem-diol tetrahedral reaction intermediate (TI) in the crystal structures. Distinctive interactions are observed for the TI binding in the active site cavity of PRWT and PRI54V. The mutant PRI54V/TI complex has lost water-mediated hydrogen bond interactions with the amides of Ile50 and Ile50' in the flap. Hence, the structures provide insight into the mechanism of drug resistance arising from this mutation. The structures also illustrate an intermediate state in the hydrolysis reaction. One of the gem-diol hydroxide groups in the PRWT complex forms a very short (2.3 A) hydrogen bond with the outer carboxylate oxygen of Asp25. Quantum chemical calculations based on this TI structure are consistent with protonation of the inner carboxylate oxygen of Asp25', in contrast to several theoretical studies. These TI complexes and quantum calculations are discussed in relation to the chemical mechanism of the peptide bond hydrolysis catalyzed by PR.
Figures


Similar articles
-
Capturing the reaction pathway in near-atomic-resolution crystal structures of HIV-1 protease.Biochemistry. 2012 Oct 2;51(39):7726-32. doi: 10.1021/bi3008092. Epub 2012 Sep 21. Biochemistry. 2012. PMID: 22963370 Free PMC article.
-
X-ray snapshot of HIV-1 protease in action: observation of tetrahedral intermediate and short ionic hydrogen bond SIHB with catalytic aspartate.J Am Chem Soc. 2010 May 12;132(18):6366-73. doi: 10.1021/ja100002b. J Am Chem Soc. 2010. PMID: 20397633
-
Effect of flap mutations on structure of HIV-1 protease and inhibition by saquinavir and darunavir.J Mol Biol. 2008 Aug 1;381(1):102-15. doi: 10.1016/j.jmb.2008.05.062. Epub 2008 Jul 1. J Mol Biol. 2008. PMID: 18597780 Free PMC article.
-
Amprenavir complexes with HIV-1 protease and its drug-resistant mutants altering hydrophobic clusters.FEBS J. 2010 Sep;277(18):3699-714. doi: 10.1111/j.1742-4658.2010.07771.x. Epub 2010 Aug 2. FEBS J. 2010. PMID: 20695887 Free PMC article.
-
The active site of HIV-1 protease.Med Res Rev. 2001 Jul;21(4):348-53. doi: 10.1002/med.1012. Med Res Rev. 2001. PMID: 11410934 Review.
Cited by
-
Structure of HIV-1 protease in complex with potent inhibitor KNI-272 determined by high-resolution X-ray and neutron crystallography.Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4641-6. doi: 10.1073/pnas.0809400106. Epub 2009 Mar 9. Proc Natl Acad Sci U S A. 2009. PMID: 19273847 Free PMC article.
-
Enzymatic and structural analysis of the I47A mutation contributing to the reduced susceptibility to HIV protease inhibitor lopinavir.Protein Sci. 2008 Sep;17(9):1555-64. doi: 10.1110/ps.036079.108. Epub 2008 Jun 17. Protein Sci. 2008. PMID: 18560011 Free PMC article.
-
In pursuit of virtual lead optimization: pruning ensembles of receptor structures for increased efficiency and accuracy during docking.Proteins. 2009 Apr;75(1):62-74. doi: 10.1002/prot.22214. Proteins. 2009. PMID: 18781587 Free PMC article.
-
Highly conserved glycine 86 and arginine 87 residues contribute differently to the structure and activity of the mature HIV-1 protease.Proteins. 2010 Mar;78(4):1015-25. doi: 10.1002/prot.22625. Proteins. 2010. PMID: 19899162 Free PMC article.
-
Mass-dependent bond vibrational dynamics influence catalysis by HIV-1 protease.J Am Chem Soc. 2011 Dec 7;133(48):19358-61. doi: 10.1021/ja209391n. Epub 2011 Nov 11. J Am Chem Soc. 2011. PMID: 22059645 Free PMC article.
References
-
- Emini EA. The Human Immunodeficiency Virus: Biology, Immunology, and Therapy. Princeton University Press; Princeton: 2002. p. 532.
-
- Ogden R. HIV Protease Inhibitors. Infect Dis Therap. 2003;30:523–553.
-
- Marks K, Gulick RM. Progress in HIV Treatment. HIV Chemotherapy. 2005:1–32.
-
- Johnson VA, Brun-Vezinet F, Clotet B, Kuritzkes DR, Pillay D, Schapiro JM, Richman DD. Update of the Drug Resistance Mutations in HIV-1: Fall 2006. Topics in HIV Medicine. 2006;14:125–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

