In silico elucidation of the molecular mechanism defining the adverse effect of selective estrogen receptor modulators
- PMID: 18052534
- PMCID: PMC2098847
- DOI: 10.1371/journal.pcbi.0030217
In silico elucidation of the molecular mechanism defining the adverse effect of selective estrogen receptor modulators
Abstract
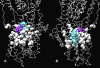
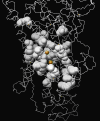
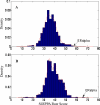
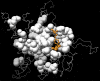
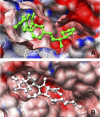
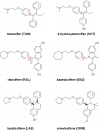
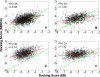
Early identification of adverse effect of preclinical and commercial drugs is crucial in developing highly efficient therapeutics, since unexpected adverse drug effects account for one-third of all drug failures in drug development. To correlate protein-drug interactions at the molecule level with their clinical outcomes at the organism level, we have developed an integrated approach to studying protein-ligand interactions on a structural proteome-wide scale by combining protein functional site similarity search, small molecule screening, and protein-ligand binding affinity profile analysis. By applying this methodology, we have elucidated a possible molecular mechanism for the previously observed, but molecularly uncharacterized, side effect of selective estrogen receptor modulators (SERMs). The side effect involves the inhibition of the Sacroplasmic Reticulum Ca2+ ion channel ATPase protein (SERCA) transmembrane domain. The prediction provides molecular insight into reducing the adverse effect of SERMs and is supported by clinical and in vitro observations. The strategy used in this case study is being applied to discover off-targets for other commercially available pharmaceuticals. The process can be included in a drug discovery pipeline in an effort to optimize drug leads and reduce unwanted side effects.
Conflict of interest statement
Figures


References
-
- Kennedy T. Managing the drug discovery/development interface. Drug Discov Today. 1997;2:436–444.
-
- Whitebread S, Hamon J, Bojanic D, Urban L. Keynote review: in vitro safety pharmacology profiling: an essential tool for successful drug development. Drug Discov Today. 2005;10:1421–1433. - PubMed
-
- Bass A, Kinter L, Williams P. Origins, practices and future of safety pharmacology. J Pharmacol Toxicol Methods. 2004;49:145–151. - PubMed
-
- MacDonald ML, Lamerdin J, Owens S, Keon BH, Bilter GK, et al. Identifying off-target effects and hidden phenotypes of drugs in human cells. Nat Chem Biol. 2007;2:329–337. - PubMed
-
- Fliri AF, Loging WT, Thaderio PF, Volkmann RA. Analysis of drug-induced effect patterns to link structure and side effects of medicines. Nat Chem Biol. 2005;1:389–397. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

