The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells
- PMID: 18053004
- PMCID: PMC10952005
- DOI: 10.1111/j.1462-5822.2007.01102.x
The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells
Abstract
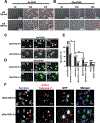
Type III secreted (T3SS) effectors are important virulence factors in acute infections caused by Pseudomonas aeruginosa. PA103, a well-studied human lung isolate, encodes and secretes two effectors, ExoU and ExoT. ExoU is a potent cytotoxin that causes necrotic cell death. In addition, PA103 can induce cell death in macrophages in an ExoU-independent but T3SS-dependent manner. We now demonstrate that ExoT is both necessary and sufficient to cause apoptosis in HeLa cells and that it activates the mitochondrial/cytochrome c-dependent apoptotic pathway. We further show that ExoT induction of cell death is primarily dependent on its ADP ribosyltransferase domain activity. Our data also indicate that the T3SS apparatus can cause necrotic cell death, which is effectively blocked by ExoT, suggesting that P. aeruginosa may have evolved strategies to prevent T3SS-induced necrosis.
Figures
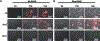





References
-
- Alaoui-El-Azher M, Jia J, Lian W, and Jin S (2006) ExoS of Pseudomonas aeruginosa induces apoptosis through a Fas receptor/caspase 8-independent pathway in HeLa cells. Cell Microbiol 8: 326–338. - PubMed
-
- Barbieri JT, and Sun J (2004) Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol 152: 79–92. - PubMed
-
- Callus BA, and Vaux DL (2007) Caspase inhibitors: viral, cellular and chemical. Cell Death Differ 14: 73–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

