Cancer stem cells: the lessons from pre-cancerous stem cells
- PMID: 18053092
- PMCID: PMC3823473
- DOI: 10.1111/j.1582-4934.2007.00170.x
Cancer stem cells: the lessons from pre-cancerous stem cells
Abstract
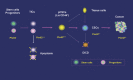
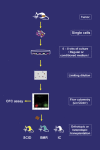
How a cancer is initiated and established remains elusive despite all the advances in decades of cancer research. Recently the cancer stem cell (CSC) hypothesis has been revived, challenging the long-standing model of "clonal evolution" for cancer development and implicating the dawning of a potential cure for cancer [1]. The recent identification of precancerous stem cells (pCSCs) in cancer, an early stage of CSC development, however, implicates that the "clonal evolution" is not contradictory to the CSC hypothesis, but is rather an aspect of the process of CSC development [2]. The discovery of pCSC has revealed and will continue to reveal the volatile properties of CSC with respects to their phenotype, differentiation and tumorigenic capacity during initiation and progression. Both pCSC and CSC might also serve as precursors of tumor stromal components such as tumor vasculogenic stem/progenitor cells (TVPCs). Thus, the CSC hypothesis covers the developing process of tumor-initiating cells (TIC) --> pCSC --> CSC --> cancer, a cellular process that should parallel the histological process of hyperplasia/metaplasia (TIC) --> precancerous lesions (pCSC) --> malignant lesions (CSC --> cancer). The embryonic stem (ES) cell and germline stem (GS) cell genes are subverted in pCSCs. Especially the GS cell protein piwil2 may play an important role during the development of TIC --> pCSC --> CSC, and this protein may be used as a common biomarker for early detection, prevention, and treatment of cancer. As cancer stem cell research is yet in its infancy, definitive conclusions regarding the role of pCSC can not be made at this time. However this review will discuss what we have learned from pCSC and how this has led to innovative ideas that may eventually have major impacts on the understanding and treatment of cancer.
Figures


References
-
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells–perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. - PubMed
-
- Chen L, Shen R, Ye Y, Pu XA, Liu X, Duan W, Wen J, Zimmerer J, Wang Y, Liu Y, Lasky LC, Heerema NA, Perrotti D, Ozato K, Kuramochi-Miyagawa S, Nakano T, Yates AJ, Carson Iii WE, Lin H, Barsky SH, Gao JX. Precancerous stem cells have the potential for both benign and malignant differentiation. PLoS ONE. 2007;2:e293. - PMC - PubMed
-
- Berman JJ, Albores-Saavedra J, Bostwick D, Delellis R, Eble J, Hamilton SR, Hruban RH, Mutter GL, Page D, Rohan T, Travis W, Henson DE. Precancer: a conceptual working definition–results of a consensus conference. Cancer Detect Prev. 2006;30:387–94. - PubMed
-
- Fearon ER, Vogelstein B. A genetic model for col-orectal tumorigenesis. Cell. 1990;61:759–67. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

