The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors
- PMID: 18053164
- PMCID: PMC2246246
- DOI: 10.1186/ar2336
The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors
Abstract
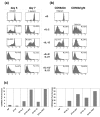
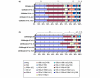
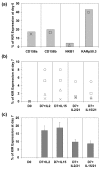
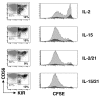
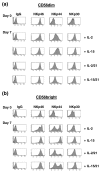
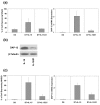
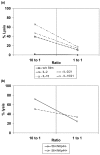
Natural killer (NK) cells play a crucial role in the immune response to micro-organisms and tumours. Recent evidence suggests that NK cells also regulate the adaptive T-cell response and that it might be possible to exploit this ability to eliminate autoreactive T cells in autoimmune disease and alloreactive T cells in transplantation. Mature NK cells consist of a highly diverse population of cells that expresses different receptors to facilitate recognition of diseased cells and possibly pathogens themselves. Ex vivo culture of NK cells with cytokines such as IL-2 and IL-15 is an approach that permits significant expansion of the NK cell subpopulations, which are likely to have potent antitumour, antiviral, or immunomodulatory effects in autoimmunity. Our data indicate that the addition of IL-21 has a synergistic effect by increasing the numbers of NK cells on a large scale. IL-2 and IL-15 may induce the expression of killer cell immunoglobulin-like receptors (KIRs) in KIR-negative populations, the c-lectin receptor NKG2D and the natural cytotoxic receptor NKp44. The addition of IL-21 to IL-15 or IL-2 can modify the pattern of the KIR receptors and inhibit NKp44 expression by reducing the expression of the adaptor DAP-12. IL-21 also preserved the production of interferon-gamma and enhanced the cytotoxic properties of NK cells. Our findings indicate that the proinflammatory cytokines IL-2, IL-15 and IL-21 can modify the peripheral repertoire of NK cells. These properties may be used to endow subpopulations of NK cells with specific phenotypes, which may be used in ex vivo cellular immunotherapy strategies.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

