Broad network-based predictability of Saccharomyces cerevisiae gene loss-of-function phenotypes
- PMID: 18053250
- PMCID: PMC2246260
- DOI: 10.1186/gb-2007-8-12-r258
Broad network-based predictability of Saccharomyces cerevisiae gene loss-of-function phenotypes
Abstract
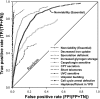
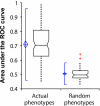
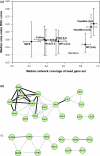
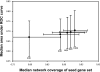
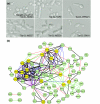
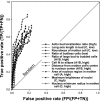
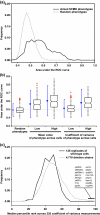
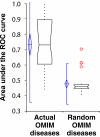
We demonstrate that loss-of-function yeast phenotypes are predictable by guilt-by-association in functional gene networks. Testing 1,102 loss-of-function phenotypes from genome-wide assays of yeast reveals predictability of diverse phenotypes, spanning cellular morphology, growth, metabolism, and quantitative cell shape features. We apply the method to extend a genome-wide screen by predicting, then verifying, genes whose disruption elongates yeast cells, and to predict human disease genes. To facilitate network-guided screens, a web server is available http://www.yeastnet.org.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

