The role of the unfolded protein response in the heart
- PMID: 18054039
- PMCID: PMC2746718
- DOI: 10.1016/j.yjmcc.2007.10.017
The role of the unfolded protein response in the heart
Abstract
The misfolding of nascent proteins, or the unfolding of proteins after synthesis is complete, can occur in response to numerous environmental stresses, or as a result of mutations that de-stabilize protein structure. Cells have developed elaborate protein quality control systems that recognize improperly folded proteins and either refold them or facilitate their degradation. One such quality control system is the unfolded protein response, or the UPR. The UPR is a highly conserved signal transduction system that is activated when cells are subjected to conditions that alter the endoplasmic reticulum (ER) in ways that impair the folding of nascent proteins in this organelle. Recent observations indicate that in the heart, the UPR is activated during acute stresses, including ischemia/reperfusion, as well as upon longer term stresses that lead to cardiac hypertrophy and heart failure. Moreover, certain aspects of the UPR are activated during, and are required for proper heart development. This review summarizes recent studies of the UPR in the heart, focusing on the possible roles of the UPR in contributing to, or protecting from ischemia/reperfusion damage.
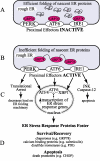
Figures
References
-
- Blobel G. Protein targeting. Biosci Rep. 2000 Oct;20(5):303–44. - PubMed
-
- Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006 Dec 8;99(12):1315–28. - PubMed
-
- Patterson C, Ike C, Willis PWt, Stouffer GA, Willis MS. The bitter end: the ubiquitin-proteasome system and cardiac dysfunction. Circulation. 2007 Mar 20;115(11):1456–63. - PubMed
-
- Drummond IA, Lee AS, Resendez E, Jr., Steinhardt RA. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1987 Sep 15;262(26):12801–5. - PubMed
-
- Lee AS. Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992 Apr;4(2):267–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

