A role for CITED2, a CBP/p300 interacting protein, in colon cancer cell invasion
- PMID: 18054336
- PMCID: PMC2211427
- DOI: 10.1016/j.febslet.2007.11.072
A role for CITED2, a CBP/p300 interacting protein, in colon cancer cell invasion
Abstract
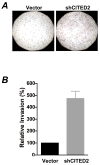
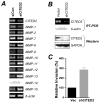
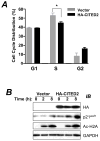
A thorough understanding of histone acetyltransferase CBP/p300-mediated regulation of gene expression and cell growth is essential to identify mechanisms relevant to the development of histone deacetylase (HDAC) inhibitor-based preventive and therapeutic strategies. We found that knockdown of CBP/p300 interacting coactivator with glutamic acid/aspartic acid-rich tail 2 (CITED2) increased colon cancer cell invasiveness in vitro. Gene expression profiling revealed that CITED2 knockdown induced matrix metalloproteinase-13 (MMP-13) gene expression in colon cancer cells. Butyrate, a naturally occurring HDAC inhibitor, induced CITED2 expression and downregulated MMP-13 expression in RKO cells. Additionally, ectopic expression of CITED2 arrested RKO cell growth. Thus, CITED2 regulates colon cancer invasion and might be a target for HDAC inhibitor-based intervention of colon cancer.
Figures




References
-
- Markowitz SD, Dawson DM, Willis J, Willson JK. Focus on colon cancer. Cancer Cell. 2002;1:233–6. - PubMed
-
- Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–603. - PubMed
-
- Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23:4225–31. - PubMed
-
- Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

