Inhibition of intestinal absorption of cholesterol by ezetimibe or bile acids by SC-435 alters lipoprotein metabolism and extends the lifespan of SR-BI/apoE double knockout mice
- PMID: 18054357
- PMCID: PMC2364648
- DOI: 10.1016/j.atherosclerosis.2007.10.012
Inhibition of intestinal absorption of cholesterol by ezetimibe or bile acids by SC-435 alters lipoprotein metabolism and extends the lifespan of SR-BI/apoE double knockout mice
Abstract
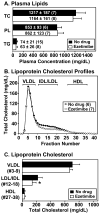
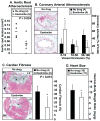
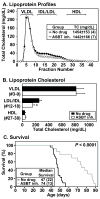
SR-BI/apoE double knockout (dKO) mice exhibit many features of human coronary heart disease (CHD), including hypercholesterolemia, occlusive coronary atherosclerosis, cardiac hypertrophy, myocardial infarctions, cardiac dysfunction and premature death. Ezetimibe is a FDA-approved, intestinal cholesterol absorption inhibitor that lowers plasma LDL cholesterol in humans and animals and inhibits aortic root atherosclerosis in apoE KO mice, but has not been proven to reduce CHD. Three-week-ezetimibe treatment of dKO mice (0.005% (w/w) in standard chow administered from weaning) resulted in a 35% decrease in cholesterol in IDL/LDL-size lipoproteins, but not in VLDL- and HDL-size lipoproteins. Ezetimibe treatment significantly reduced aortic root (57%) and coronary arterial (68%) atherosclerosis, cardiomegaly (24%) and cardiac fibrosis (57%), and prolonged the lives of the mice (27%). This represents the first demonstration of beneficial effects of ezetimibe treatment on CHD. The dKO mice were similarly treated with SC-435 (0.01% (w/w)), an apical sodium codependent bile acid transporter (ASBT) inhibitor, that blocks intestinal absorption of bile acids, lowers plasma cholesterol in animals, and reduces aortic root atherosclerosis in apoE KO mice. The effects of SC-435 treatment were similar to those of ezetimibe: 37% decrease in ILD/LDL-size lipoprotein cholesterol and 57% prolongation in median lifespan. Thus, inhibition of intestinal absorption of either cholesterol (ezetimibe) or bile acids (SC-435) significantly reduced plasma IDL/LDL-size lipoprotein cholesterol levels and improved survival of SR-BI/apoE dKO mice. The SR-BI/apoE dKO murine model of atherosclerotic occlusive, arterial CHD appears to provide a useful system to evaluate compounds that modulate cholesterol homeostasis and atherosclerosis.
Figures

References
-
- Kreisberg RA, Oberman A. Clinical review 141: lipids and atherosclerosis: lessons learned from randomized controlled trials of lipid lowering and other relevant studies. J Clin Endocrinol Metab. 2002;87:423–437. - PubMed
-
- Burnett JR, Huff MW. Cholesterol absorption inhibitors as a therapeutic option for hypercholesterolaemia. Expert Opin Investig Drugs. 2006;15:1337–1351. - PubMed
-
- Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, Sybertz EJ, Davis HR., Jr In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283:157–163. - PubMed
-
- Rosenblum SB, Huynh T, Afonso A, Davis HR, Jr, Yumibe N, Clader JW, Burnett DA. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4 -hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem. 1998;41:973–980. - PubMed
-
- Gazi IF, Mikhailidis DP. Non-low-density lipoprotein cholesterol-associated actions of ezetimibe: an overview. Expert Opin Ther Targets. 2006;10:851–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

