Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation
- PMID: 18054552
- PMCID: PMC2185546
- DOI: 10.1053/j.gastro.2007.09.005
Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation
Abstract
Background & aims: In addition to its role as the primary mediator of the enteroinsular axis, glucose-dependent insulinotropic polypeptide (GIP) may play a critical role in the development of obesity. The purpose of these studies was to characterize the effects of GIP and its receptor (GIPR) in adipocyte development and signaling.
Methods: Effects of GIP and GIPR on differentiated 3T3-L1 cells were analyzed using Western blot analysis, Oil-Red-O staining, cyclic adenosine monophosphate radioimmunoassay, immunofluorescence microscopy, and glucose uptake measurements.
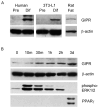
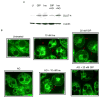
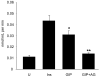
Results: To determine whether GIP and GIPR are important components in adipocyte development, the expression profile of GIPR during differentiation was examined. GIPR protein expression was enhanced during the differentiation process, and coincubation with its ligand GIP augmented the expression of aP2, a fat cell marker. Conversely, the suppression of GIPR expression by a specific short hairpin RNA attenuated Oil-Red-O staining and aP2 expression, suggesting that the GIPR may play a critical role in adipocyte development. To investigate specific signaling components that may mediate the effects of GIP, we analyzed Akt, glucose transporter-4, and glucose uptake, all of which are modulated by insulin in fat cells. Like insulin, GIP induced the activation of Akt in a concentration-dependent manner, promoted membrane glucose transporter-4 accumulation, and enhanced [(3)H]-2-deoxyglucose uptake.
Conclusions: These studies provide further evidence for an important physiologic role for GIP in lipid homeostasis and possibly in the pathogenesis of obesity. Furthermore, our data indicate that the GIPR might represent a suitable target for the treatment of obesity.
Figures


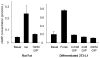



Similar articles
-
Glucose-dependent insulinotropic peptide impairs insulin signaling via inducing adipocyte inflammation in glucose-dependent insulinotropic peptide receptor-overexpressing adipocytes.FASEB J. 2012 Jun;26(6):2383-93. doi: 10.1096/fj.11-196782. Epub 2012 Feb 24. FASEB J. 2012. PMID: 22366643
-
Resistin knockout mice exhibit impaired adipocyte glucose-dependent insulinotropic polypeptide receptor (GIPR) expression.Diabetes. 2013 Feb;62(2):471-7. doi: 10.2337/db12-0257. Epub 2012 Sep 21. Diabetes. 2013. PMID: 23002036 Free PMC article.
-
Functional expression of glucose-dependent insulinotropic polypeptide receptors is coupled to differentiation in a human adipocyte model.Int J Obes (Lond). 2008 Nov;32(11):1705-11. doi: 10.1038/ijo.2008.148. Epub 2008 Sep 9. Int J Obes (Lond). 2008. PMID: 18779825
-
The pathogenic role of the GIP/GIPR axis in human endocrine tumors: emerging clinical mechanisms beyond diabetes.Rev Endocr Metab Disord. 2020 Mar;21(1):165-183. doi: 10.1007/s11154-019-09536-6. Rev Endocr Metab Disord. 2020. PMID: 31933128 Review.
-
Glucose-dependent insulinotropic polypeptide (GIP) receptor antagonists as anti-diabetic agents.Peptides. 2018 Feb;100:173-181. doi: 10.1016/j.peptides.2017.11.021. Peptides. 2018. PMID: 29412817 Review.
Cited by
-
Onset of effects of testosterone treatment and time span until maximum effects are achieved.Eur J Endocrinol. 2011 Nov;165(5):675-85. doi: 10.1530/EJE-11-0221. Epub 2011 Jul 13. Eur J Endocrinol. 2011. PMID: 21753068 Free PMC article. Review.
-
The transcription factor hepatocyte nuclear factor 4A acts in the intestine to promote white adipose tissue energy storage.Nat Commun. 2022 Jan 11;13(1):224. doi: 10.1038/s41467-021-27934-w. Nat Commun. 2022. PMID: 35017517 Free PMC article.
-
Pharmacological and non-pharmacological interventions to influence adipose tissue function.Cardiovasc Diabetol. 2011 Jan 28;10:13. doi: 10.1186/1475-2840-10-13. Cardiovasc Diabetol. 2011. PMID: 21276223 Free PMC article. Review.
-
The role of incretins in glucose homeostasis and diabetes treatment.Pharmacol Rev. 2008 Dec;60(4):470-512. doi: 10.1124/pr.108.000604. Epub 2008 Dec 12. Pharmacol Rev. 2008. PMID: 19074620 Free PMC article. Review.
-
Glucose-dependent insulinotropic polypeptide-mediated signaling pathways enhance apical PepT1 expression in intestinal epithelial cells.Am J Physiol Gastrointest Liver Physiol. 2015 Jan 1;308(1):G56-62. doi: 10.1152/ajpgi.00168.2014. Epub 2014 Nov 6. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25377315 Free PMC article.
References
-
- Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22:39–47. - PubMed
-
- Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37:826–8. - PubMed
-
- Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–42. - PubMed
-
- Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4. discussion 4-5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

