CD40L disruption enhances Abeta vaccine-mediated reduction of cerebral amyloidosis while minimizing cerebral amyloid angiopathy and inflammation
- PMID: 18055209
- PMCID: PMC2743124
- DOI: 10.1016/j.nbd.2007.09.009
CD40L disruption enhances Abeta vaccine-mediated reduction of cerebral amyloidosis while minimizing cerebral amyloid angiopathy and inflammation
Abstract
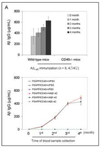
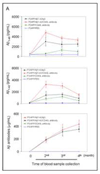
Amyloid-beta (Abeta) immunization efficiently reduces amyloid plaque load and memory impairment in transgenic mouse models of Alzheimer's disease (AD). Active Abeta immunization has also yielded favorable results in a subset of AD patients. However, a small percentage of patients developed severe aseptic meningoencephalitis associated with brain inflammation and infiltration of T-cells. We have shown that blocking the CD40-CD40 ligand (L) interaction mitigates Abeta-induced inflammatory responses and enhances Abeta clearance. Here, we utilized genetic and pharmacologic approaches to test whether CD40-CD40L blockade could enhance the efficacy of Abeta(1-42) immunization, while limiting potentially damaging inflammatory responses. We show that genetic or pharmacologic interruption of the CD40-CD40L interaction enhanced Abeta(1-42) immunization efficacy to reduce cerebral amyloidosis in the PSAPP and Tg2576 mouse models of AD. Potentially deleterious pro-inflammatory immune responses, cerebral amyloid angiopathy (CAA) and cerebral microhemorrhage were reduced or absent in these combined approaches. Pharmacologic blockade of CD40L decreased T-cell neurotoxicity to Abeta-producing neurons. Further reduction of cerebral amyloidosis in Abeta-immunized PSAPP mice completely deficient for CD40 occurred in the absence of Abeta immunoglobulin G (IgG) antibodies or efflux of Abeta from brain to blood, but was rather correlated with anti-inflammatory cytokine profiles and reduced plasma soluble CD40L. These results suggest CD40-CD40L blockade promotes anti-inflammatory cellular immune responses, likely resulting in promotion of microglial phagocytic activity and Abeta clearance without generation of neurotoxic Abeta-reactive T-cells. Thus, combined approaches of Abeta immunotherapy and CD40-CD40L blockade may provide for a safer and more effective Abeta vaccine.
Figures
















Similar articles
-
Lack of P-glycoprotein Results in Impairment of Removal of Beta-Amyloid and Increased Intraparenchymal Cerebral Amyloid Angiopathy after Active Immunization in a Transgenic Mouse Model of Alzheimer's Disease.Curr Alzheimer Res. 2017;14(6):656-667. doi: 10.2174/1567205013666161201201227. Curr Alzheimer Res. 2017. PMID: 27915995
-
Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice.Stem Cells Dev. 2008 Jun;17(3):423-39. doi: 10.1089/scd.2008.0018. Stem Cells Dev. 2008. PMID: 18366296 Free PMC article.
-
Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer's disease mouse model in the absence of an Abeta-specific cellular immune response.J Neurosci. 2006 May 3;26(18):4717-28. doi: 10.1523/JNEUROSCI.0381-06.2006. J Neurosci. 2006. PMID: 16672644 Free PMC article.
-
Anti-Aβ Antibodies and Cerebral Amyloid Angiopathy Complications.Front Immunol. 2019 Jul 4;10:1534. doi: 10.3389/fimmu.2019.01534. eCollection 2019. Front Immunol. 2019. PMID: 31333665 Free PMC article. Review.
-
Developing novel immunogens for a safe and effective Alzheimer's disease vaccine.Prog Brain Res. 2009;175:83-93. doi: 10.1016/S0079-6123(09)17506-4. Prog Brain Res. 2009. PMID: 19660650 Free PMC article. Review.
Cited by
-
Microglial priming of antigen presentation and adaptive stimulation in Alzheimer's disease.Cell Mol Life Sci. 2019 Oct;76(19):3681-3694. doi: 10.1007/s00018-019-03132-2. Epub 2019 May 15. Cell Mol Life Sci. 2019. PMID: 31093687 Free PMC article. Review.
-
Acquired immunity and Alzheimer's disease.J Biomed Res. 2022 Jul 28;37(1):15-29. doi: 10.7555/JBR.36.20220083. J Biomed Res. 2022. PMID: 36165328 Free PMC article.
-
How to get from here to there: macrophage recruitment in Alzheimer's disease.Curr Alzheimer Res. 2011 Mar;8(2):156-63. doi: 10.2174/156720511795256017. Curr Alzheimer Res. 2011. PMID: 21345166 Free PMC article. Review.
-
Alternative Abeta immunotherapy approaches for Alzheimer's disease.CNS Neurol Disord Drug Targets. 2009 Apr;8(2):114-27. doi: 10.2174/187152709787847306. CNS Neurol Disord Drug Targets. 2009. PMID: 19355932 Free PMC article. Review.
-
SOLUBLE CD40 LIGAND IN DEMENTIA.Drugs Future. 2009 Apr 1;34(4):333-340. doi: 10.1358/dof.2009.034.04.1358595. Drugs Future. 2009. PMID: 19777117 Free PMC article.
References
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–919. - PubMed
-
- Bernhardi RV, Nicholls JG. Transformation of leech microglial cell morphology and properties following co-culture with injured central nervous system tissue. J Exp Biol. 1999;202(Pt 6):723–728. - PubMed
-
- Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 2003;14:297–309. - PubMed
-
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. - PubMed
-
- Calingasan NY, Erdely HA, Altar CA. Identification of CD40 ligand in Alzheimer's disease and in animal models of Alzheimer's disease and brain injury. Neurobiol Aging. 2002;23:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

