Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate
- PMID: 18055472
- PMCID: PMC2564802
- DOI: 10.1136/ard.2007.080002
Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate
Abstract
Objectives: This double-blind trial evaluated the efficacy and safety of abatacept or infliximab vs placebo. The primary objective of this study was to evaluate the mean change from baseline in Disease Activity Score (based on erythrocyte sedimentation rates; DAS28 (ESR)) for the abatacept vs placebo groups at day 197.
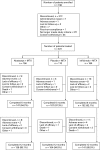
Methods: Patients with rheumatoid arthritis (RA) and an inadequate response to methotrexate (MTX) were randomised 3:3:2 to abatacept ( approximately 10 mg/kg every 4 weeks, n = 156), infliximab (3 mg/kg every 8 weeks, n = 165), or placebo (every 4 weeks, n = 110) and background MTX. Safety and efficacy were assessed throughout the study.
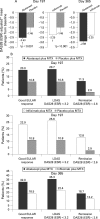
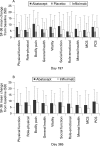
Results: Similar patient demographics and clinical characteristics were present at baseline between groups, with mean scores of approximately 1.7 for HAQ-DI and 6.8 for DAS28 (ESR). At 6 months, mean changes in DAS28 (ESR) were significantly greater for abatacept vs placebo (-2.53 vs -1.48, p<0.001) and infliximab vs placebo (-2.25 vs -1.48, p<0.001). For abatacept vs infliximab treatment at day 365, reductions in the DAS28 (ESR) were -2.88 vs -2.25. At day 365, the following response rates were observed for abatacept and infliximab, respectively: American College of Rheumatology (ACR) 20, 72.4 and 55.8%; ACR 50, 45.5 and 36.4%; ACR 70, 26.3 and 20.6%; low disease activity score (LDAS), 35.3 and 22.4%; DAS28-defined remission, 18.7 and 12.2%; good European League Against Rheumatism (EULAR) responses, 32.0 and 18.5%; and Health Assessment Questionnaire Disability Index (HAQ-DI), 57.7 and 52.7%. Mean changes in physical component summary (PCS) were 9.5 and 7.6, and mental component summary (MCS) were 6.0 and 4.0, for abatacept and infliximab, respectively. Over 1 year, adverse events (AEs) (89.1 vs 93.3%), serious AEs (SAEs) (9.6 vs 18.2%), serious infections (1.9 vs 8.5%) and discontinuations due to AEs (3.2 vs 7.3%) and SAEs (2.6 vs 3.6%) were lower with abatacept than infliximab.
Conclusions: In this study, abatacept and infliximab (3 mg/kg every 8 weeks) demonstrated similar efficacy. Overall, abatacept had a relatively more acceptable safety and tolerability profile, with fewer SAEs, serious infections, acute infusional events and discontinuations due to AEs than the infliximab group.
Trial registration number: NCT00095147.
Conflict of interest statement
Figures

Comment in
-
How do the efficacy and safety of abatacept and infliximab compare in the treatment of active RA?Nat Clin Pract Rheumatol. 2009 Mar;5(3):126-7. doi: 10.1038/ncprheum1022. Nat Clin Pract Rheumatol. 2009. PMID: 19252515
References
-
- Yamada A, Salama AD, Sayegh MH. The role of novel T cell costimulatory pathways in autoimmunity and transplantation. J Am Soc Nephrol 2002;13:559–75 - PubMed
-
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 2006;144:865–76 - PubMed
-
- Genovese M, Becker J-C, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 2005;353:1114–23 - PubMed
-
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24 - PubMed
-
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41:1552–63 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

