New point of care Chlamydia Rapid Test--bridging the gap between diagnosis and treatment: performance evaluation study
- PMID: 18055487
- PMCID: PMC2128659
- DOI: 10.1136/bmj.39402.463854.AE
New point of care Chlamydia Rapid Test--bridging the gap between diagnosis and treatment: performance evaluation study
Abstract
Objective: To evaluate the performance of a new Chlamydia Rapid Test with vaginal swab specimens as a potential tool for chlamydia diagnosis and screening.
Design: Performance evaluation study. Settings A young people's sexual health centre (site 1) and two genitourinary medicine clinics (sites 2 and 3) in the United Kingdom.
Participants: 1349 women aged between 16 and 54 attending one of the three clinics.
Main outcome measures: Sensitivity, specificity, positive predictive value, and negative predictive value of the Chlamydia Rapid Test versus polymerase chain reaction and strand displacement amplification assays; correlation between the Chlamydia Rapid Test visual signal and organism load; acceptability to participants of self collected vaginal swabs as the specimen type for Chlamydia testing.
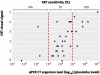
Results: Polymerase chain reaction positivity rates for Chlamydia trachomatis infection were 8.4% (56/663) at site 1, 9.4% (36/385) at site 2, and 6.0% (18/301) at site 3. Compared with polymerase chain reaction assay, the resolved sensitivity, specificity, positive predictive value, and negative predictive value of the Chlamydia Rapid Test were 83.5% (91/109), 98.9% (1224/1238), 86.7% (91/105), and 98.6% (1224/1242). Compared with strand displacement amplification assay, sensitivity and specificity of the Chlamydia Rapid Test were 81.6% (40/49) and 98.3% (578/588). Organism load of self collected vaginal swabs ranged from 5.97x10(2) to 1.09x10(9) Chlamydia plasmids per swab, which correlated well with the Chlamydia Rapid Test's visual signal (r=0.6435, P<0.0001). Most (95.9%) surveyed participants felt comfortable about collecting their own swabs.
Conclusions: The performance of the Chlamydia Rapid Test with self collected vaginal swabs indicates that it would be an effective same day diagnostic and screening tool for Chlamydia infection in women. The availability of Chlamydia Rapid Test results within 30 minutes allows for immediate treatment and contact tracing, potentially reducing the risks of persistent infection and onward transmission. It could also provide a simple and reliable alternative to nucleic acid amplification tests in chlamydia screening programmes.
Conflict of interest statement
Competing interests: JJW and HHL are equity holders of a spin-off company, Diagnostics for the Real World, based on the rapid test technologies developed at the University of Cambridge. Both the University of Cambridge and the Wellcome Trust are also equity holders of the company.
Figures

Comment in
-
Chlamydia Rapid Test was moderately accurate for diagnosing Chlamydia infection in women.Evid Based Nurs. 2008 Jul;11(3):89. doi: 10.1136/ebn.11.3.89. Evid Based Nurs. 2008. PMID: 18583501 No abstract available.
Similar articles
-
Performance evaluation of a new rapid urine test for chlamydia in men: prospective cohort study.BMJ. 2009 Jul 28;339:b2655. doi: 10.1136/bmj.b2655. BMJ. 2009. PMID: 19638650 Free PMC article.
-
Evaluation of a new amplified enzyme immunoassay (EIA) for the detection of Chlamydia trachomatis in male urine, female endocervical swab, and patient obtained vaginal swab specimens.J Clin Pathol. 2000 May;53(5):350-4. doi: 10.1136/jcp.53.5.350. J Clin Pathol. 2000. PMID: 10889816 Free PMC article. Clinical Trial.
-
The vaginal introitus: a novel site for Chlamydia trachomatis testing in women.Am J Obstet Gynecol. 1996 May;174(5):1542-6. doi: 10.1016/s0002-9378(96)70603-8. Am J Obstet Gynecol. 1996. PMID: 9065126
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.Health Technol Assess. 2007 Mar;11(8):iii-iv, ix-xii, 1-165. doi: 10.3310/hta11080. Health Technol Assess. 2007. PMID: 17311735 Review.
-
Diagnosis of urogenital Chlamydia trachomatis infection by use of DNA amplification.APMIS Suppl. 1999;89:5-36. APMIS Suppl. 1999. PMID: 10189834 Review.
Cited by
-
Applying new technologies for diagnosing sexually transmitted infections in resource-poor settings.Sex Transm Infect. 2011 Dec;87 Suppl 2(Suppl 2):ii28-30. doi: 10.1136/sti.2010.047647. Sex Transm Infect. 2011. PMID: 22110150 Free PMC article. No abstract available.
-
A multiplexed microfluidic PCR assay for sensitive and specific point-of-care detection of Chlamydia trachomatis.PLoS One. 2012;7(12):e51685. doi: 10.1371/journal.pone.0051685. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23272140 Free PMC article.
-
What's the Point? How Point-of-Care STI Tests Can Impact Infected Patients.Point Care. 2010 Mar 1;9(1):36-46. doi: 10.1097/POC.0b013e3181d2d8cc. Point Care. 2010. PMID: 20401167 Free PMC article.
-
Highly sensitive and novel point-of-care system, aQcare Chlamydia TRF kit for detecting Chlamydia trachomatis by using europium (Eu) (III) chelated nanoparticles.Ann Lab Med. 2015 Jan;35(1):50-6. doi: 10.3343/alm.2015.35.1.50. Epub 2014 Dec 8. Ann Lab Med. 2015. PMID: 25553280 Free PMC article.
-
Diagnostic accuracy of a prototype point-of-care test for ocular Chlamydia trachomatis under field conditions in The Gambia and Senegal.PLoS Negl Trop Dis. 2011 Aug;5(8):e1234. doi: 10.1371/journal.pntd.0001234. Epub 2011 Aug 2. PLoS Negl Trop Dis. 2011. PMID: 21829735 Free PMC article.
References
-
- Cates W, Wasserheit JN. Genital chlamydial infections: epidemiology and reproductive sequelae. Am J Obstetr Gynecol 1991;164:1771-81. - PubMed
-
- Paavonen J, Lehtinen M. Chlamydial pelvic inflammatory disease. Hum Reprod Update 1996;2:519-29. - PubMed
-
- National Chlamydia Screening Programme Steering Group. New frontiers: annual report of the national chlamydia screening programme in England 2005/06 London: Health Protection Agency, 2006. (available at www.hpa.org.uk/publications/2006/ncsp/).
-
- Davies SC, Otto B, Partohudoyo S, Chrisnadarmani VAMA, Neilsen GA, Ciaffi L, et al. Sexually transmitted infections among female sex workers in Kupang, Indonesia. Sex Transm Dis 2003;30:671-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
