International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states
- PMID: 18055507
- PMCID: PMC2517428
- DOI: 10.1124/pr.107.07108
International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states
Abstract
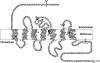
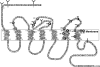
The mammalian bombesin receptor family comprises three G protein-coupled heptahelical receptors: the neuromedin B (NMB) receptor (BB(1)), the gastrin-releasing peptide (GRP) receptor (BB(2)), and the orphan receptor bombesin receptor subtype 3 (BRS-3) (BB(3)). Each receptor is widely distributed, especially in the gastrointestinal (GI) tract and central nervous system (CNS), and the receptors have a large range of effects in both normal physiology and pathophysiological conditions. The mammalian bombesin peptides, GRP and NMB, demonstrate a broad spectrum of pharmacological/biological responses. GRP stimulates smooth muscle contraction and GI motility, release of numerous GI hormones/neurotransmitters, and secretion and/or hormone release from the pancreas, stomach, colon, and numerous endocrine organs and has potent effects on immune cells, potent growth effects on both normal tissues and tumors, potent CNS effects, including regulation of circadian rhythm, thermoregulation; anxiety/fear responses, food intake, and numerous CNS effects on the GI tract as well as the spinal transmission of chronic pruritus. NMB causes contraction of smooth muscle, has growth effects in various tissues, has CNS effects, including effects on feeding and thermoregulation, regulates thyroid-stimulating hormone release, stimulates various CNS neurons, has behavioral effects, and has effects on spinal sensory transmission. GRP, and to a lesser extent NMB, affects growth and/or differentiation of various human tumors, including colon, prostate, lung, and some gynecologic cancers. Knockout studies show that BB(3) has important effects in energy balance, glucose homeostasis, control of body weight, lung development and response to injury, tumor growth, and perhaps GI motility. This review summarizes advances in our understanding of the biology/pharmacology of these receptors, including their classification, structure, pharmacology, physiology, and role in pathophysiological conditions.
Figures


References
-
- Acs P, Beheshti M, Szallasi Z, Li L, Yuspa SH, Blumberg PM. Effect of a Tyrosine 155 to Phenylalanine Mutation of Protein Kinase Cδ on the Proliferative and Tumorigenic Properties of NIH 3T3 Fibroblasts. Carcinogenesis. 2000;21:887–891. - PubMed
-
- Akeson M, Sainz E, Mantey SA, Jensen RT, Battey JF. Identification of Four Amino Acids in the Gastrin-Releasing Peptide C Receptor That Are Required for High Affinity Agonist Binding. J Biol Chem. 1997;272:17405–17409. - PubMed
-
- Ally RA, Ives KL, Traube E, Eltounsi I, Chen PW, Cahill PJ, Battey JF, Hellmich MR, Kroog GS. Agonistand Protein Kinase C-Induced Phosphorylation Have Similar Functional Consequences for Gastrin-Releasing Peptide Receptor Signaling Via Gq. Mol Pharmacol. 2003;64:890–904. - PubMed
-
- Alptekin N, Yagci RV, Ertan A, Jiang NY, Rice JC, Sbeiti M, Rossowski WJ, Coy DH. Comparison of Prolonged in Vivo Inhibitory Activity of Several Potent Bombesin (BN) Antagonists on BN-Stimulated Amylase Secretion in the Rat. Peptides. 1991;12:749–753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

