Oligomerization of membrane-bound diphtheria toxin (CRM197) facilitates a transition to the open form and deep insertion
- PMID: 18055530
- PMCID: PMC2257914
- DOI: 10.1529/biophysj.107.113498
Oligomerization of membrane-bound diphtheria toxin (CRM197) facilitates a transition to the open form and deep insertion
Abstract
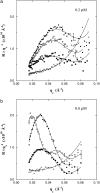
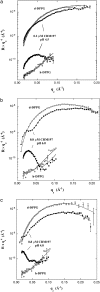
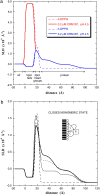
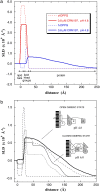
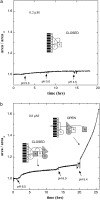
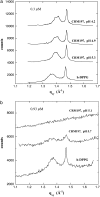
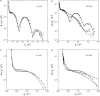
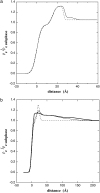
Diphtheria toxin (DT) contains separate domains for receptor-specific binding, translocation, and enzymatic activity. After binding to cells, DT is taken up into endosome-like acidic compartments where the translocation domain inserts into the endosomal membrane and releases the catalytic domain into the cytosol. The process by which the catalytic domain is translocated across the endosomal membrane is known to involve pH-induced conformational changes; however, the molecular mechanisms are not yet understood, in large part due to the challenge of probing the conformation of the membrane-bound protein. In this work neutron reflection provided detailed conformational information for membrane-bound DT (CRM197) in situ. The data revealed that the bound toxin oligomerizes with increasing DT concentration and that the oligomeric form (and only the oligomeric form) undergoes a large extension into solution with decreasing pH that coincides with deep insertion of residues into the membrane. We interpret the large extension as a transition to the open form. These results thus indicate that as a function of bulk DT concentration, adsorbed DT passes from an inactive state with a monomeric dimension normal to the plane of the membrane to an active state with a dimeric dimension normal to the plane of the membrane.
Figures



References
-
- Chenal, A., P. Nizard, and D. Gillet. 2002. Structure and function of diphtheria toxin: from pathology to engineering. J. Toxicol. Toxin Rev. 21:321–358.
-
- Collier, R. 2001. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon. 39:1793–1803. - PubMed
-
- Sandvig, K., and B. van Deurs. 2002. Membrane traffic exploited by protein toxins. Annu. Rev. Cell Dev. Biol. 18:1–24. - PubMed
-
- Madshus, I. H., and H. Stenmark. 1992. Entry of ADP-ribosylating toxins into cells. Curr. Top. Microbiol. Immunol. 175:1–26. - PubMed
-
- Pappenheimer, A. M. Jr. 1977. Diphtheria toxin. Annu. Rev. Biochem. 46:69–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

