Measuring and modeling chloride-hydroxyl exchange in the Guinea-pig ventricular myocyte
- PMID: 18055536
- PMCID: PMC2257879
- DOI: 10.1529/biophysj.107.118885
Measuring and modeling chloride-hydroxyl exchange in the Guinea-pig ventricular myocyte
Abstract
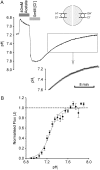
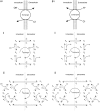
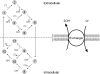
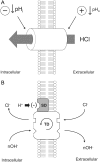
Protons are powerful modulators of cardiac function. Their intracellular concentration is regulated by sarcolemmal ion transporters that export or import H+-ions (or their ionic equivalent: HCO3-, OH-). One such transporter, which imports H+-equivalents, is a putative Cl-/OH- exchanger (CHE). A strong candidate for CHE is SLC26A6 protein, a product of the SLC26A gene family of anion transporters, which has been detected in murine heart. SLC26A6 protein is suggested to be an electrogenic 1Cl-/2OH-(2HCO3-) exchanger. Unfortunately, there is insufficient characterization of cardiac CHE against which the properties of heterologously expressed SLC26A6 can be matched. We therefore investigated the proton, Cl-, and voltage dependence of CHE activity in guinea-pig ventricular myocytes, using voltage-clamp, intracellular pH fluorescence, and mathematical modeling techniques. We find that CHE activity is tightly regulated by intracellular and extracellular pH, is voltage-insensitive over a wide range (+/-80 mV), and displays substrate dependence suggestive of electroneutral 1Cl-/1OH- exchange. These properties exclude electrogenic SLC26A6 as sole contributor to CHE. Either the SLC26A6 product in heart is electroneutral, or CHE comprises at least two transporters with oppositely balanced voltage sensitivity. Alternatively, CHE may comprise an H+-Cl- coinflux system, which cannot be distinguished kinetically from an exchanger. Irrespective of ionic mechanism, CHE's pH sensitivity helps to define resting intracellular pH, and hence basal function in the heart.
Figures





References
-
- Komukai, K., F. Brette, C. Pascarel, and C. H. Orchard. 2002. Electrophysiological response of rat ventricular myocytes to acidosis. Am. J. Physiol. Heart Circ. Physiol. 283:H412–H422. - PubMed
-
- Poole, R. C., and A. P. Halestrap. 1993. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol. Cell Physiol. 264:C761–C782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

