The monocyte chemoattractant protein-1/cognate CC chemokine receptor 2 system affects cell motility in cultured human podocytes
- PMID: 18055544
- PMCID: PMC2111103
- DOI: 10.2353/ajpath.2007.070398
The monocyte chemoattractant protein-1/cognate CC chemokine receptor 2 system affects cell motility in cultured human podocytes
Abstract
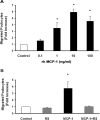
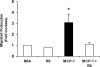
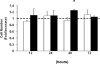
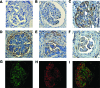
In crescentic glomerulonephritis (GN), monocyte chemoattractant protein-1 (MCP-1) is overexpressed within the glomeruli, and MCP-1 blockade has renoprotective effects. Adult podocytes are in a quiescent state, but acquisition of a migratory/proliferative phenotype has been described in crescentic GN and implicated in crescent formation. The cognate CC chemokine receptor 2 (CCR2), the MCP-1 receptor, is expressed by other cell types besides monocytes and has been implicated in both cell proliferation and migration. We investigated whether MCP-1 binding to CCR2 can induce a migratory/proliferative response in cultured podocytes. MCP-1 binding to CCR2 enhanced podocyte chemotaxis/haptotaxis in a concentration-dependent manner and had a modest effect on cell proliferation. Closure of a wounded podocyte monolayer was delayed by CCR2 blockade, and CCR2 was overexpressed at the wound edge, suggesting a role for CCR2 in driving podocyte migration. Immunohistochemical analysis of kidney biopsies from patients with crescentic GN demonstrated CCR2 expression in both podocytes and cellular crescents, confirming the clinical relevance of our in vitro findings. In conclusion, the MCP-1/CCR2 system is functionally active in podocytes and may be implicated in the migratory events triggered by podocyte injury in crescentic GN and other glomerular diseases.
Figures



References
-
- Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. - PubMed
-
- Le Hir M, Keller C, Eschmann V, Hähnel B, Hosser H, Kriz W. Podocyte bridges between the tuft and Bowman’s capsule: an early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2001;12:2060–2071. - PubMed
-
- Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol. 2004;15:61–67. - PubMed
-
- Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–34832. - PubMed
-
- Kakizaki Y, Waga S, Sugimoto K, Tanaka H, Nukii K, Takeya M, Yoshimura T, Yokoyama M. Production of monocyte chemoattractant protein-1 by bovine glomerular endothelial cells. Kidney Int. 1995;48:1866–1874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

