Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16
- PMID: 18055583
- PMCID: PMC2245851
- DOI: 10.1104/pp.107.112748
Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16
Abstract
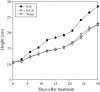
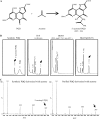
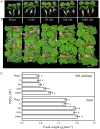
Pseudomonas fluorescens B16 is a plant growth-promoting rhizobacterium. To determine the factors involved in plant growth promotion by this organism, we mutagenized wild-type strain B16 using OmegaKm elements and isolated one mutant, K818, which is defective in plant growth promotion, in a rockwool culture system. A cosmid clone, pOK40, which complements the mutant K818, was isolated from a genomic library of the parent strain. Tn3-gusA mutagenesis of pOK40 revealed that the genes responsible for plant growth promotion reside in a 13.3-kb BamHI fragment. Analysis of the DNA sequence of the fragment identified 11 putative open reading frames, consisting of seven known and four previously unidentified pyrroloquinoline quinone (PQQ) biosynthetic genes. All of the pqq genes showed expression only in nutrient-limiting conditions in a PqqH-dependent manner. Electrospray ionization-mass spectrometry analysis of culture filtrates confirmed that wild-type B16 produces PQQ, whereas mutants defective in plant growth promotion do not. Application of wild-type B16 on tomato (Solanum lycopersicum) plants cultivated in a hydroponic culture system significantly increased the height, flower number, fruit number, and total fruit weight, whereas none of the strains that did not produce PQQ promoted tomato growth. Furthermore, 5 to 1,000 nm of synthetic PQQ conferred a significant increase in the fresh weight of cucumber (Cucumis sativus) seedlings, confirming that PQQ is a plant growth promotion factor. Treatment of cucumber leaf discs with PQQ and wild-type B16 resulted in the scavenging of reactive oxygen species and hydrogen peroxide, suggesting that PQQ acts as an antioxidant in plants.
Figures




References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215 403–410 - PubMed
-
- Bashan Y, Levanony H (1991) Alterations in membrane potential and in efflux in plant roots induced by Azospirillum brasilense. Plant Soil 137 99–103
-
- Bloemberg GV, Lutenberg BJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Microbiol 4 343–350 - PubMed
-
- Bonas U, Stall RE, Staskawicz BJ (1989) Genetic and structural characterization of the avirulence gene avrBs3 from Xanthomonas campestris pv. vesicatoria. Mol Gen Genet 218 127–136 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

