A viroid RNA with a specific structural motif inhibits chloroplast development
- PMID: 18055612
- PMCID: PMC2174877
- DOI: 10.1105/tpc.106.049775
A viroid RNA with a specific structural motif inhibits chloroplast development
Abstract
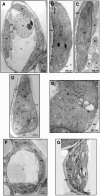
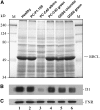
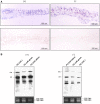
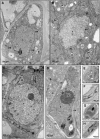
Peach latent mosaic viroid (PLMVd) is a chloroplast-replicating RNA that propagates in its natural host, peach (Prunus persica), as a complex mixture of variants, some of which are endowed with specific structural and pathogenic properties. This is the case of variant PC-C40, with an insertion of 12 to 13 nucleotides that folds into a hairpin capped by a U-rich loop, which is responsible for an albino-variegated phenotype known as peach calico (PC). We have applied a combination of ultrastructural, biochemical, and molecular approaches to dissect the pathogenic effects of PC-C40. Albino sectors of leaves infected with variant PC-C40 presented palisade cells that did not completely differentiate into a columnar layer and altered plastids with irregular shape and size and with rudimentary thylakoids, resembling proplastids. Furthermore, impaired processing and accumulation of plastid rRNAs and, consequently, of the plastid translation machinery was observed in the albino sectors of leaves infected with variant PC-C40 but not in the adjacent green areas or in leaves infected by mosaic-inducing or latent variants (including PC-C40Delta, in which the 12- to 13-nucleotide insertion was deleted). Protein gel blot and RT-PCR analyses showed that the altered plastids support the import of nucleus-encoded proteins, including a chloroplast RNA polymerase, the transcripts of which were detected. RNA gel blot and in situ hybridizations revealed that PLMVd replicates in the albino leaf sectors and that it can invade the shoot apical meristem and induce alterations in proplastids, bypassing the RNA surveillance system that restricts the entry of a nucleus-replicating viroid and most RNA viruses. Therefore, a non-protein-coding RNA with a specific structural motif can interfere with an early step of the chloroplast developmental program, leading ultimately to an albino-variegated phenotype resembling that of certain variegated mutants in which plastid rRNA maturation is also impaired. Our results highlight the potential of viroids for further dissection of RNA trafficking and pathogenesis in plants.
Figures



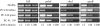


Similar articles
-
Small RNAs containing the pathogenic determinant of a chloroplast-replicating viroid guide the degradation of a host mRNA as predicted by RNA silencing.Plant J. 2012 Jun;70(6):991-1003. doi: 10.1111/j.1365-313X.2012.04940.x. Epub 2012 Apr 4. Plant J. 2012. PMID: 22332758
-
How sequence variants of a plastid-replicating viroid with one single nucleotide change initiate disease in its natural host.RNA Biol. 2019 Jul;16(7):906-917. doi: 10.1080/15476286.2019.1600396. Epub 2019 Apr 16. RNA Biol. 2019. PMID: 30990352 Free PMC article.
-
Molecular and Biological Characteristics of a Peach Latent Mosaic Viroid PC Isolate in Peach from China: Base Mutations in Hairpin Stems and Implications for Symptomatology.Plant Dis. 2024 Jul;108(7):2181-2189. doi: 10.1094/PDIS-11-23-2454-RE. Epub 2024 Jul 1. Plant Dis. 2024. PMID: 38522091
-
Viroid pathogenesis: a critical appraisal of the role of RNA silencing in triggering the initial molecular lesion.FEMS Microbiol Rev. 2020 May 1;44(3):386-398. doi: 10.1093/femsre/fuaa011. FEMS Microbiol Rev. 2020. PMID: 32379313 Review.
-
Viroids: unusual small pathogenic RNAs.Acta Biochim Pol. 2004;51(3):587-607. Acta Biochim Pol. 2004. PMID: 15448723 Review.
Cited by
-
On the early identification and characterization of pear blister canker viroid, apple dimple fruit viroid, peach latent mosaic viroid and chrysanthemum chlorotic mottle viroid.Virus Res. 2023 Jan 2;323:199012. doi: 10.1016/j.virusres.2022.199012. Epub 2022 Nov 24. Virus Res. 2023. PMID: 36436691 Free PMC article.
-
Thermotherapy Followed by Shoot Tip Cryotherapy Eradicates Latent Viruses and Apple Hammerhead Viroid from In Vitro Apple Rootstocks.Plants (Basel). 2022 Feb 22;11(5):582. doi: 10.3390/plants11050582. Plants (Basel). 2022. PMID: 35270052 Free PMC article.
-
Viroids, and the Legacy of Ricardo Flores (1947-2020).Cells. 2021 Sep 28;10(10):2570. doi: 10.3390/cells10102570. Cells. 2021. PMID: 34685550 Free PMC article.
-
Viroid replication: rolling-circles, enzymes and ribozymes.Viruses. 2009 Sep;1(2):317-34. doi: 10.3390/v1020317. Epub 2009 Sep 14. Viruses. 2009. PMID: 21994552 Free PMC article.
-
A Short Indel-Lacking-Resistance Gene Triggers Silencing of the Photosynthetic Machinery Components Through TYLCSV-Associated Endogenous siRNAs in Tomato.Front Plant Sci. 2018 Oct 11;9:1470. doi: 10.3389/fpls.2018.01470. eCollection 2018. Front Plant Sci. 2018. PMID: 30364213 Free PMC article.
References
-
- Ambrós, S., Hernández, C., and Flores, R. (1999). Rapid generation of genetic heterogeneity in progenies from individual cDNA clones of peach latent mosaic viroid in its natural host. J. Gen. Virol. 80 2239–2252. - PubMed
-
- Barba, M., Cupidi, A., Loreti, S., Faggioli, F., and Martino, L. (1995). In vitro micrografting: A technique to eliminate peach latent mosaic viroid from peach. Acta Hortic. 386 531–535.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

