Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat
- PMID: 18056009
- PMCID: PMC2853251
- DOI: 10.1177/1545968307308550
Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat
Abstract
Objective: The myelin protein Nogo inhibits axon regeneration by binding to its receptor (NgR) on axons. Intrathecal delivery of an NgR antagonist (NEP1-40) promotes growth of injured corticospinal axons and recovery of motor function following a dorsal hemisection. The authors used a similar design to examine recovery and repair after a lesion that interrupts the rubrospinal tract (RST).
Methods: Rats received a lateral funiculotomy at C4 and NEP1-40 or vehicle was delivered to the cervical spinal cord for 4 weeks. Outcome measures included motor and sensory tests and immunohistochemistry.
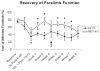
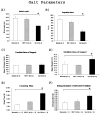
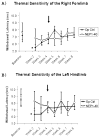
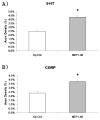
Results: Gait analysis showed recovery in the NEP1-40-treated group compared to operated controls, and a test of forelimb usage also showed a beneficial effect. The density of labeled RST axons increased ipsilaterally in the NEP1-40 group in the lateral funiculus rostral to the lesion and contralaterally in both gray and white matter. Thus, rubrospinal axons exhibited diminished dieback and/or growth up to the lesion site. This was accompanied by greater density of 5HT and calcitonin gene-related peptide axons adjacent to and into the lesion/matrix site in the NEP1-40 group.
Conclusions: NgR blockade after RST injury is associated with axonal growth and/or diminished dieback of severed RST axons up to but not into or beyond the lesion/matrix site, and growth of serotonergic and dorsal root axons adjacent to and into the lesion/matrix site. NgR blockade also supported partial recovery of function. The authors' results indicate that severed rubrospinal axons respond to NEP1-40 treatment but less robustly than corticospinal, raphe-spinal, or dorsal root axons.
Figures







References
-
- Hunt D, Coffin RS, Anderson PN. The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review. J Neurocytol. 2002;31:93–120. - PubMed
-
- Chen MS, Huber AB, van der Haar ME, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. - PubMed
-
- Prinjha R, Moore SE, Vinson M, et al. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

