OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats
- PMID: 18056162
- PMCID: PMC2916741
- DOI: 10.1093/brain/awm267
OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats
Abstract
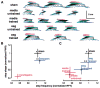
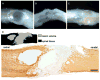
Numerous treatment strategies for spinal cord injury seek to maximize recovery of function and two strategies that show substantial promise are olfactory bulb-derived olfactory ensheathing glia (OEG) transplantation and treadmill step training. In this study we re-examined the issue of the effectiveness of OEG implantation but used objective, quantitative measures of motor performance to test if there is a complementary effect of long-term step training and olfactory bulb-derived OEG implantation. We studied complete mid-thoracic spinal cord transected adult female rats and compared four experimental groups: media-untrained, media-trained, OEG-untrained and OEG-trained. To assess the extent of hindlimb locomotor recovery at 4 and 7 months post-transection we used three quantitative measures of stepping ability: plantar stepping performance until failure, joint movement shape and movement frequency compared to sham controls. OEG transplantation alone significantly increased the number of plantar steps performed at 7 months post-transection, while training alone had no effect at either time point. Only OEG-injected rats plantar placed their hindpaws for more than two steps by the 7-month endpoint of the study. OEG transplantation combined with training resulted in the highest percentage of spinal rats per group that plantar stepped, and was the only group to significantly improve its stepping abilities between the 4- and 7-month evaluations. Additionally, OEG transplantation promoted tissue sparing at the transection site, regeneration of noradrenergic axons and serotonergic axons spanning the injury site. Interestingly, the caudal stump of media- and OEG-injected rats contained a similar density of serotonergic axons and occasional serotonin-labelled interneurons. These data demonstrate that olfactory bulb-derived OEG transplantation improves hindlimb stepping in paraplegic rats and further suggest that task-specific training may enhance this OEG effect.
Figures



Comment in
-
Step training with severely damaged spinal cord.Brain. 2009 Jul;132(Pt 7):e117; author reply e118. doi: 10.1093/brain/awn341. Epub 2009 Jan 13. Brain. 2009. PMID: 19141493 No abstract available.
Similar articles
-
Further evidence of olfactory ensheathing glia facilitating axonal regeneration after a complete spinal cord transection.Exp Neurol. 2011 May;229(1):109-19. doi: 10.1016/j.expneurol.2011.01.007. Epub 2011 Jan 25. Exp Neurol. 2011. PMID: 21272578 Free PMC article.
-
Noradrenergic innervation of the rat spinal cord caudal to a complete spinal cord transection: effects of olfactory ensheathing glia.Exp Neurol. 2010 Mar;222(1):59-69. doi: 10.1016/j.expneurol.2009.12.008. Epub 2009 Dec 16. Exp Neurol. 2010. PMID: 20025875 Free PMC article.
-
Axon regeneration can facilitate or suppress hindlimb function after olfactory ensheathing glia transplantation.J Neurosci. 2011 Mar 16;31(11):4298-310. doi: 10.1523/JNEUROSCI.4967-10.2011. J Neurosci. 2011. PMID: 21411671 Free PMC article.
-
Olfactory ensheathing glia: their contribution to primary olfactory nervous system regeneration and their regenerative potential following transplantation into the injured spinal cord.Brain Res Rev. 2007 Nov;56(1):236-58. doi: 10.1016/j.brainresrev.2007.07.013. Epub 2007 Aug 14. Brain Res Rev. 2007. PMID: 17884174 Review.
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
Cited by
-
Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial.Brain. 2008 Sep;131(Pt 9):2376-86. doi: 10.1093/brain/awn173. Epub 2008 Aug 8. Brain. 2008. PMID: 18689435 Free PMC article. Clinical Trial.
-
Taking a bite out of spinal cord injury: do dental stem cells have the teeth for it?Cell Mol Life Sci. 2016 Apr;73(7):1413-37. doi: 10.1007/s00018-015-2126-5. Epub 2016 Jan 14. Cell Mol Life Sci. 2016. PMID: 26768693 Free PMC article. Review.
-
Transplantation-mediated strategies to promote axonal regeneration following spinal cord injury.Respir Physiol Neurobiol. 2009 Nov 30;169(2):171-82. doi: 10.1016/j.resp.2009.07.016. Epub 2009 Aug 7. Respir Physiol Neurobiol. 2009. PMID: 19665611 Free PMC article. Review.
-
A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury.J Neurotrauma. 2012 May 20;29(8):1600-13. doi: 10.1089/neu.2011.2199. Epub 2012 Apr 18. J Neurotrauma. 2012. PMID: 22401139 Free PMC article.
-
Olfactory Ensheathing Cell Transplantation after a Complete Spinal Cord Transection Mediates Neuroprotective and Immunomodulatory Mechanisms to Facilitate Regeneration.J Neurosci. 2016 Jun 8;36(23):6269-86. doi: 10.1523/JNEUROSCI.0085-16.2016. J Neurosci. 2016. PMID: 27277804 Free PMC article.
References
-
- Barbeau H, Chau C, Rossignol S. Noradrenergic agonists and locomotor training affect locomotor recovery after cord transection in adult cats. Brain Res Bull. 1993;30:387–93. - PubMed
-
- Barbeau H, Rossignol S. Enhancement of locomotor recovery following spinal cord injury. Curr Opin Neurol. 1994;7:517–24. - PubMed
-
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. - PubMed
-
- Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–60. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed

