Intracellular metabolism of the nucleotide prodrug GS-9131, a potent anti-human immunodeficiency virus agent
- PMID: 18056281
- PMCID: PMC2224749
- DOI: 10.1128/AAC.01209-07
Intracellular metabolism of the nucleotide prodrug GS-9131, a potent anti-human immunodeficiency virus agent
Abstract
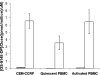
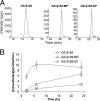
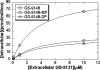
GS-9131 is a phosphonoamidate prodrug of the novel ribose-modified phosphonate nucleotide analog GS-9148 that demonstrates potent anti-human immunodeficiency virus type 1 (HIV-1) activity and an excellent resistance profile in vitro. Prodrug moieties were optimized for the efficient delivery of GS-9148 and its active diphosphate (DP) metabolite to lymphoid cells following oral administration. To understand the intracellular pharmacology of GS-9131, incubations were performed with various types of lymphoid cells in vitro. The intracellular accumulation and antiviral activity levels of GS-9148 were limited by its lack of cellular permeation, and GS-9131 increased the delivery of GS-9148-DP by 76- to 290-fold relative to that of GS-9148. GS-9131 activation was saturable at high extracellular concentrations, potentially due to a high-affinity promoiety cleavage step. Once inside the cells, GS-9148 was efficiently phosphorylated, forming similar amounts of anabolites in primary lymphoid cells. The levels of GS-9148-DP formed in peripheral blood mononuclear cells infected with HIV-1 were similar to that in uninfected PBMCs, and approximately equivalent intracellular concentrations of GS-9148-DP and tenofovir (TVF)-DP were required to inhibit viral replication by 90%. Once it was formed, GS-9148-DP was efficiently retained in activated CD4(+) cells, with a half-life of 19 h. In addition, GS-9131 showed a low potential for drug interactions with other adenine nucleoside/nucleotide reverse transcriptase inhibitors, based on the lack of competition for anabolism between suprapharmacologic concentrations of GS-9148 and TVF and the lack of activity of GS-9131 metabolites against purine nucleoside phosphorylase, an enzyme involved in the clearance of 2',3'-dideoxyinosine. Together, these observations elucidate the cellular pharmacology of GS-9131 and illustrate its efficient loading of lymphoid cells, resulting in a prolonged intracellular exposure to the active metabolite GS-9148-DP.
Figures




References
-
- Beauchamp, L. M., J. V. Tuttle, M. E. Rodriguez, and M. L. Sznaidman. 1996. Guanine, pyrazolo[3,4-d]pyrimidine, and triazolo[4,5-d]pyrimidine (8-azaguanine) phosphonate acyclic derivatives as inhibitors of purine nucleoside phosphorylase. J. Med. Chem. 39:949-956. - PubMed
-
- Becher, F., R. Landman, S. Mboup, C. N. Kane, A. Canestri, F. Liegeois, M. Vray, M. H. Prevot, G. Leleu, and H. Benech. 2004. Monitoring of didanosine and stavudine intracellular trisphosphorylated anabolite concentrations in HIV-infected patients. AIDS 18:181-187. - PubMed
-
- Birkus, G., R. Wang, X. Liu, N. Kutty, H. MacArthur, T. Cihlar, C. Gibbs, S. Swaminathan, W. Lee, and M. McDermott. 2007. Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob. Agents Chemother. 51:543-550. - PMC - PubMed
-
- Cihlar, T., A. S. Ray, C. G. Boojamra, L. Zhang, H. Hui, G. Laflamme, J. E. Vela, D. Grant, J. Chen, F. Myrick, K. L. White, Y. Gao, K.-Y. Lin, J. L. Douglas, N. T. Parkin, A. Carey, R. Pakdaman, and R. L. Mackman. 2008. Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob. Agents Chemother. 52:655-665. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

