Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131
- PMID: 18056282
- PMCID: PMC2224772
- DOI: 10.1128/AAC.01215-07
Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131
Abstract
GS-9148 [(5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2-yloxymethyl)phosphonic acid] is a novel ribose-modified human immunodeficiency virus type 1 (HIV-1) nucleotide reverse transcriptase (RT) inhibitor (NRTI) selected from a series of nucleoside phosphonate analogs for its favorable in vitro biological properties including (i) a low potential for mitochondrial toxicity, (ii) a minimal cytotoxicity in renal proximal tubule cells and other cell types, (iii) synergy in combination with other antiretrovirals, and (iv) a unique resistance profile against multiple NRTI-resistant HIV-1 strains. Notably, antiviral resistance analysis indicated that neither the K65R, L74V, or M184V RT mutation nor their combinations had any effect on the antiretroviral activity of GS-9148. Viruses carrying four or more thymidine analog mutations showed a substantially smaller change in GS-9148 activity relative to that observed with most marketed NRTIs. GS-9131, an ethylalaninyl phosphonoamidate prodrug designed to maximize the intracellular delivery of GS-9148, is a potent inhibitor of multiple subtypes of HIV-1 clinical isolates, with a mean 50% effective concentration of 37 nM. Inside cells, GS-9131 is readily hydrolyzed to GS-9148, which is further phosphorylated to its active diphosphate metabolite (A. S. Ray, J. E. Vela, C. G. Boojamra, L. Zhang, H. Hui, C. Callebaut, K. Stray, K.-Y. Lin, Y. Gao, R. L. Mackman, and T. Cihlar, Antimicrob. Agents Chemother. 52:648-654, 2008). GS-9148 diphosphate acts as a competitive inhibitor of RT with respect to dATP (K(i) = 0.8 muM) and exhibits low inhibitory potency against host polymerases including DNA polymerase gamma. Oral administration of GS-9131 to beagle dogs at a dose of 3 mg/kg of body weight resulted in high and persistent levels of GS-9148 diphosphate in peripheral blood mononuclear cells (with a maximum intracellular concentration of >9 microM and a half-life of >24 h). This favorable preclinical profile makes GS-9131 an attractive clinical development candidate for the treatment of patients infected with NRTI-resistant HIV.
Figures
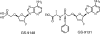
Similar articles
-
Intracellular metabolism of the nucleotide prodrug GS-9131, a potent anti-human immunodeficiency virus agent.Antimicrob Agents Chemother. 2008 Feb;52(2):648-54. doi: 10.1128/AAC.01209-07. Epub 2007 Dec 3. Antimicrob Agents Chemother. 2008. PMID: 18056281 Free PMC article.
-
Discovery of GS-9131: Design, synthesis and optimization of amidate prodrugs of the novel nucleoside phosphonate HIV reverse transcriptase (RT) inhibitor GS-9148.Bioorg Med Chem. 2010 May 15;18(10):3606-17. doi: 10.1016/j.bmc.2010.03.041. Epub 2010 Mar 27. Bioorg Med Chem. 2010. PMID: 20409721
-
Activation of 9-[(R)-2-[[(S)-[[(S)-1-(Isopropoxycarbonyl)ethyl]amino] phenoxyphosphinyl]-methoxy]propyl]adenine (GS-7340) and other tenofovir phosphonoamidate prodrugs by human proteases.Mol Pharmacol. 2008 Jul;74(1):92-100. doi: 10.1124/mol.108.045526. Epub 2008 Apr 22. Mol Pharmacol. 2008. PMID: 18430788
-
An introduction to nucleoside and nucleotide analogues.Antivir Ther. 2001;6 Suppl 3:1-14. Antivir Ther. 2001. PMID: 11678469 Review.
-
Anti-HIV Nucleoside Phosphonate GS-9148 and Its Prodrug GS-9131: Scale Up of a 2'-F Modified Cyclic Nucleoside Phosphonate and Synthesis of Selected Amidate Prodrugs.Curr Protoc Nucleic Acid Chem. 2014 Mar 26;56:14.10.1-21. doi: 10.1002/0471142700.nc1410s56. Curr Protoc Nucleic Acid Chem. 2014. PMID: 25606977 Review.
Cited by
-
Small-molecule inhibition of human immunodeficiency virus type 1 infection by virus capsid destabilization.J Virol. 2011 Jan;85(1):542-9. doi: 10.1128/JVI.01406-10. Epub 2010 Oct 20. J Virol. 2011. PMID: 20962083 Free PMC article.
-
Novel nucleotide human immunodeficiency virus reverse transcriptase inhibitor GS-9148 with a low nephrotoxic potential: characterization of renal transport and accumulation.Antimicrob Agents Chemother. 2009 Jan;53(1):150-6. doi: 10.1128/AAC.01183-08. Epub 2008 Nov 10. Antimicrob Agents Chemother. 2009. PMID: 19001108 Free PMC article.
-
Tenofovir diphosphate concentrations and prophylactic effect in a macaque model of rectal simian HIV transmission.J Antimicrob Chemother. 2014 Sep;69(9):2470-6. doi: 10.1093/jac/dku162. Epub 2014 May 26. J Antimicrob Chemother. 2014. PMID: 24862094 Free PMC article.
-
Intracellular Activation of Tenofovir Alafenamide and the Effect of Viral and Host Protease Inhibitors.Antimicrob Agents Chemother. 2015 Oct 26;60(1):316-22. doi: 10.1128/AAC.01834-15. Print 2016 Jan. Antimicrob Agents Chemother. 2015. PMID: 26503655 Free PMC article.
-
Long-Acting Anti-HIV Drugs Targeting HIV-1 Reverse Transcriptase and Integrase.Pharmaceuticals (Basel). 2019 Apr 20;12(2):62. doi: 10.3390/ph12020062. Pharmaceuticals (Basel). 2019. PMID: 31010004 Free PMC article. Review.
References
-
- Bethell, R. C., Y. S. Lie, and N. T. Parkin. 2005. In vitro activity of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor (NRTI), against 215 HIV-1 isolates resistant to other NRTIs. Antivir. Chem. Chemother. 16:295-302. - PubMed
-
- Birkus, G., R. Wang, X. Liu, N. Kutty, H. MacArthur, T. Cihlar, C. Gibbs, S. Swaminathan, W. Lee, and M. McDermott. 2007. Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob. Agents Chemother. 51:543-550. - PMC - PubMed
-
- Boojamra, C. G., R. L. Mackman, D. Y. Markevitch, V. Prasad, A. S. Ray, J. Douglas, D. Grant, C. U. Kim, and T. Cihlar. Bioorg. Med. Chem. Lett., in press. - PubMed
-
- Carr, A. 2003. Toxicity of antiretroviral therapy and implications for drug development. Nat. Rev. Drug Discov. 2:624-634. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

